Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo- and Photodynamic Therapy
Abstract
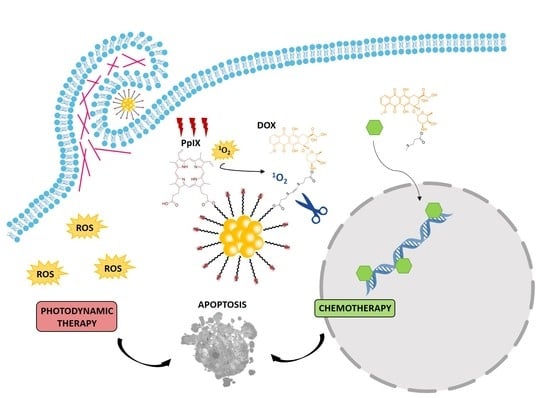
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Drugs and Nanoconjugates
2.3. Photodynamic Treatments
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.5. Neutral Red (NR) and Hoechst-33258 (H-33258) Staining
2.6. Subcellular Localization
2.7. Cellular Uptake Analysis by Flow Cytometry
2.8. ƴ-H2AX Indirect Immunofluorescence Assay
2.9. Transmission Electron Microscopy
2.10. Western Blot for Cleaved Caspase-3
2.11. Optical Microscopy
3. Results and Discussion
3.1. Synthesis and Characterization of the AuNCs
3.2. AuNCs-PpIX-DOX Design Achieves a Synergistic Effect of Photo- and Chemotherapy in the Inactivation of Human Tumoral Cells
3.3. AuNC-PpIX-DOX Treatment Triggers Massive Apoptotic Cell Death
3.4. AuNC-PpIX-DOX Complexes are Internalized through Macropinocytosis Mechanism
3.5. Attachment to AuNCs Increases PpIX Accumulation inside Tumor Cells
3.6. Attachment of DOX to AuNCs Prevents DOX-Induced Double-Strand Breaks in HeLa Nuclei
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.C.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Obaid, G.; Broekgaarden, M.; Bulin, A.L.; Huang, H.C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [Green Version]
- Blasi, M.A.; Pagliara, M.M.; Lanza, A.; Sammarco, M.G.; Caputo, C.G.; Grimaldi, G.; Scupola, A. Photodynamic Therapy in Ocular Oncology. Biomedicines 2018, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Jamison, A.; Cauchi, P.; Gilmour, D.F. Photodynamic Therapy for Circumscribed Choroidal Haemangioma in a Scottish Cohort. Ocul. Oncol. Pathol. 2018, 4, 322–330. [Google Scholar] [CrossRef]
- De Oliveira, E.C.; da Motta, V.R.; Pantoja, P.C.; Ilha, C.S.d.O.; Magalhães, R.F.; Galadari, H.; Leonardi, G.R. Actinic keratosis–review for clinical practice. Int. J. Dermatol. 2018, 58, 400–407. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Abate, L.E.; Yosipovitch, G.; Friedman, A.J. A Systematic Review of Evidence-Based Treatments for Prurigo Nodularis. J. Am. Acad. Dermatol. 2018, 80, 756–764. [Google Scholar] [CrossRef]
- Zhao, W.; Guan, M.; Nong, X.; Li, Q.; Chen, Z. The safety and efficacy of daylight photodynamic therapy in the treatment of actinic keratoses: A systematic review and meta-analysis. Int. J. Dermatol. 2018, 58, 159–166. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic therapy: Current status and future directions. Med. Princ. Pract. 2015, 24, 14–28. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Spring, B.Q.; Rizvi, I.; Xu, N.; Hasan, T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem. Photobiol. Sci. 2015, 14, 1476–1491. [Google Scholar] [CrossRef] [Green Version]
- Goler-Baron, V.; Assaraf, Y.G. Overcoming multidrug resistance via photodestruction of ABCG2-rich extracellular vesicles sequestering photosensitive chemotherapeutics. PLoS ONE 2012, 7, e35487. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Mallidi, S.; Liu, J.; Chiang, C.T.; Mai, Z.; Goldschmidt, R.; Ebrahim-Zadeh, N.; Rizvi, I.; Hasan, T. Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer. Cancer Res. 2016, 76, 1066–1077. [Google Scholar] [CrossRef] [Green Version]
- Spring, B.Q.; Sears, R.B.; Zheng, L.Z.; Mai, Z.; Watanabe, R.; Sherwood, M.E.; Schoenfeld, D.A.; Pogue, B.W.; Pereira, S.P.; Villa, E. A photoactivable multi-inhibitor nanoliposome for tumour control and simultaneous inhibition of treatment escape pathways. Nat. Nanotechnol. 2016, 11, 378. [Google Scholar] [CrossRef]
- Tangutoori, S.; Spring, B.Q.; Mai, Z.; Palanisami, A.; Mensah, L.B.; Hasan, T. Simultaneous delivery of cytotoxic and biologic therapeutics using nanophotoactivatable liposomes enhances treatment efficacy in a mouse model of pancreatic cancer. Nanomed-Nanotechnol. 2016, 12, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Lamch, Ł.; Pucek, A.; Kulbacka, J.; Chudy, M.; Jastrzębska, E.; Tokarska, K.; Bułka, M.; Brzózka, Z.; Wilk, K.A. Recent progress in the engineering of multifunctional colloidal nanoparticles for enhanced photodynamic therapy and bioimaging. Adv. Colloid Interface Sci. 2018, 261, 62–81. [Google Scholar] [CrossRef]
- Vankayala, R.; Hwang, K.C. Near-infrared-light-activatable nanomaterial-mediated phototheranostic nanomedicines: An emerging paradigm for cancer treatment. Adv. Mater. 2018, 30, 1706320. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Miranda, D.; Lovell, J.F. Chemophototherapy: An emerging treatment option for solid tumors. Adv. Sci. 2017, 4, 1600106. [Google Scholar] [CrossRef] [Green Version]
- Aftab, S.; Shah, A.; Nadhman, A.; Kurbanoglu, S.; Ozkan, S.A.; Dionysiou, D.D.; Shukla, S.S.; Aminabhavi, T.M. Nanomedicine: An effective tool in cancer therapy. Int. J. Pharm. 2018, 540, 132–149. [Google Scholar] [CrossRef]
- Kong, F.; Zhang, J.; Li, R.; Wang, Z.; Wang, W.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [Green Version]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef]
- Baugh, S.D.; Yang, Z.; Leung, D.K.; Wilson, D.M.; Breslow, R. Cyclodextrin dimers as cleavable carriers of photodynamic sensitizers. J. Am. Chem. Soc. 2001, 123, 12488–12494. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Ackerson, C.J.; Jadzinsky, P.D.; Kornberg, R.D. Thiolate ligands for synthesis of water-soluble gold clusters. J. Am. Chem. Soc. 2005, 127, 6550–6551. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Abilash, V.; Pichiah, P.T.; Arunachalam, S. Molecular mechanism of Doxorubicin-induced cardiomyopathy–An update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.S.; Burgeiro, A.; Garcia, R.; Moreno, A.J.; Carvalho, R.A.; Oliveira, P.J. Doxorubicin-induced cardiotoxicity: From bioenergetic failure and cell death to cardiomyopathy. Med. Res. Rev. 2014, 34, 106–135. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Rello, S.; Stockert, J.C.; Moreno, V.; Gamez, A.; Pacheco, M.; Juarranz, A.; Canete, M.; Villanueva, A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. [Google Scholar] [CrossRef]
- Rello-Varona, S.; Herrero-Martin, D.; Lopez-Alemany, R.; Munoz-Pinedo, C.; Tirado, O.M. “(Not) all (dead) things share the same breath”: Identification of cell death mechanisms in anticancer therapy. Cancer Res. 2015, 75, 913–917. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.J.; Cheon, Y.K.; Lee, E.J.; Lee, T.Y.; Shim, C.S. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver 2014, 8, 318–323. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Li, H.; Li, C.; Ding, H.; Zhang, M.; Guo, Y.; Sun, M. Near-infrared light triggered liposomes combining photodynamic and chemotherapy for synergistic breast tumor therapy. Colloids Surf. B Biointerfaces 2018, 173, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Bednarski, P. Evaluation for Synergistic Effects by Combinations of Photodynamic Therapy (PDT) with Temoporfin (mTHPC) and Pt (II) Complexes Carboplatin, Cisplatin or Oxaliplatin in a Set of Five Human Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekgaarden, M.; Rizvi, I.; Bulin, A.; Petrovic, L.; Goldschmidt, R.; Massodi, I.; Celli, J.P.; Hasan, T. Neoadjuvant photodynamic therapy augments immediate and prolonged oxaliplatin efficacy in metastatic pancreatic cancer organoids. Oncotarget 2018, 9, 13009. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-González, R.; Milán, P.; Bresolí-Obach, R.; Stockert, J.; Villanueva, A.; Cañete, M.; Nonell, S. Photodynamic synergistic effect of pheophorbide A and Doxorubicin in combined treatment against tumoral cells. Cancers 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99. [Google Scholar] [CrossRef]
- Chen, D.; Monteiro-Riviere, N.A.; Zhang, L.W. Intracellular imaging of quantum dots, gold, and iron oxide nanoparticles with associated endocytic pathways. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9. [Google Scholar] [CrossRef]
- Kerr, M.C.; Teasdale, R.D. Defining macropinocytosis. Traffic 2009, 10, 364–371. [Google Scholar] [CrossRef]
- He, B.; Yang, D.; Qin, M.; Zhang, Y.; He, B.; Dai, W.; Wang, X.; Zhang, Q.; Zhang, H.; Yin, C. Increased cellular uptake of peptide-modified PEGylated gold nanoparticles. Biochem. Biophys. Res. Commun. 2017, 494, 339–345. [Google Scholar] [CrossRef]
- Ding, L.; Yao, C.; Yin, X.; Li, C.; Huang, Y.; Wu, M.; Wang, B.; Guo, X.; Wang, Y.; Wu, M. Size, Shape, and Protein Corona Determine Cellular Uptake and Removal Mechanisms of Gold Nanoparticles. Small 2018, 14, e1801451. [Google Scholar] [CrossRef]
- Brandenberger, C.; Mühlfeld, C.; Ali, Z.; Lenz, A.; Schmid, O.; Parak, W.J.; Gehr, P.; Rothen-Rutishauser, B. Quantitative evaluation of cellular uptake and trafficking of plain and polyethylene glycol-coated gold nanoparticles. Small 2010, 6, 1669–1678. [Google Scholar] [CrossRef]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013, 497, 633. [Google Scholar] [CrossRef] [Green Version]
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front. Endocrinol. 2017, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- Tajiri, H.; Uruno, T.; Shirai, T.; Takaya, D.; Matsunaga, S.; Setoyama, D.; Watanabe, M.; Kukimoto-Niino, M.; Oisaki, K.; Ushijima, M. Targeting Ras-driven cancer cell survival and invasion through selective inhibition of DOCK1. Cell Rep. 2017, 19, 969–980. [Google Scholar] [CrossRef] [Green Version]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar]






| Samples | [PpIX] (mM) | [DOX] (mM) | [AuNC] (mg/mL) | Diameter [nm] | Zeta Potential [mV] |
|---|---|---|---|---|---|
| AuNCs | - | - | 15 | 1.5 ± 0.5 | −2 |
| AuNC-PpIX | 2.5 | - | 15 | 1.5 ± 0.5 | −5 |
| AuNC-DOX | - | 0.75 | 15 | 1.5 ± 0.5 | −2 |
| AuNC-PpIX-DOXx | 1.3 | 0.8 | 15 | 1.5 ± 0.5 | −3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabero, A.; Planas, O.; Gallavardin, T.; Nieves, I.; Nonell, S.; Villanueva, A. Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo- and Photodynamic Therapy. Nanomaterials 2020, 10, 2474. https://doi.org/10.3390/nano10122474
Tabero A, Planas O, Gallavardin T, Nieves I, Nonell S, Villanueva A. Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo- and Photodynamic Therapy. Nanomaterials. 2020; 10(12):2474. https://doi.org/10.3390/nano10122474
Chicago/Turabian StyleTabero, Andrea, Oriol Planas, Thibault Gallavardin, Ingrid Nieves, Santi Nonell, and Angeles Villanueva. 2020. "Smart Dual-Functionalized Gold Nanoclusters for Spatio-Temporally Controlled Delivery of Combined Chemo- and Photodynamic Therapy" Nanomaterials 10, no. 12: 2474. https://doi.org/10.3390/nano10122474