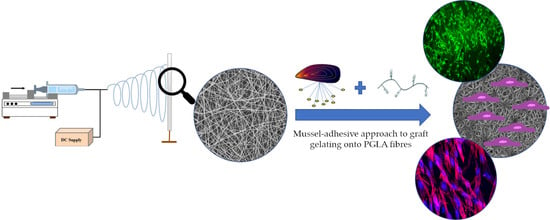
PLGA Membranes Functionalized with Gelatin through Biomimetic Mussel-Inspired Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fibrous Membrane Preparation
2.2.2. Gelatin Functionalization of Fibrous Membranes Mediated by Mussel-Inspired Coating
2.2.3. QCM-D Analysis
2.2.4. Scanning Electron Microscopy (SEM) analysis
2.2.5. Static Contact Angle Analysis
2.2.6. Infrared Analysis in ATR Modality (ATR-FTIR)
2.2.7. Differential Scanning Calorimetry (DSC)
2.2.8. Gel Permeation Chromatography (GPC)
2.2.9. Raman Spectroscopy
2.2.10. Mechanical Tensile Tests
2.2.11. Kaiser Test
2.2.12. Cell Culture and Seeding Protocol
2.2.13. Biological Tests
2.2.14. Statistical Analysis
3. Results
3.1. Characterization of the Polydopa Coating
3.2. Characterization of Membrane Functionalization with Gelatin
3.3. Cell Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Drew, C.; Lee, S.-H.; Senecal, K.J.; Kumar, J.; Samuelson, L.A. Electrospun Nanofibrous Membranes for Highly Sensitive Optical Sensors. Nano Lett. 2002, 2, 1273–1275. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Cipriani, E.; Gnavi, S.; Chiono, V.; Mattu, C.; Gentile, P.; Perroteau, I.; Zanetti, M.; Ciardelli, G. Crosslinked gelatin nanofibres: Preparation, characterisation and in vitro studies using glial-like cells. Mater. Sci. Eng. C 2013, 33, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Di Meglio, F.; Mozetic, P.; Giannitelli, S.M.; Carmagnola, I.; Castaldo, C.; Nurzynska, D.; Sacco, A.M.; Miraglia, R.; Montagnani, S.; et al. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS ONE 2018, 13, e0199896. [Google Scholar] [CrossRef] [PubMed]
- Ciardelli, G.; Chiono, V.; Vozzi, G.; Pracella, M.; Ahluwalia, A.; Barbani, N.; Cristallini, A.C.; Giusti†, P. Blends of Poly-(ε-caprolactone) and Polysaccharides in Tissue Engineering Applications. Biomacromolecules 2005, 6, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Vozzi, G.; D’Acunto, M.; Brinzi, S.; Domenici, C.; Vozzi, F.; Ahluwalia, A.; Barbani, N.; Giusti, P.; Ciardelli, G. Characterisation of blends between poly(ε-caprolactone) and polysaccharides for tissue engineering applications. Mater. Sci. Eng. C 2009, 29, 2174–2187. [Google Scholar] [CrossRef]
- Chiono, V.; Ciardelli, G.; Vozzi, G.; Cortez, J.; Barbani, N.; Gentile, P.; Giusti, P. Enzymatically-Modified Melt-Extruded Guides for Peripheral Nerve Repair. Eng. Life Sci. 2008, 8, 226–237. [Google Scholar] [CrossRef]
- Schnell, E.; Klinkhammer, K.; Balzer, S.; Brook, G.; Klee, D.; Dalton, P.; Mey, J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-ε-caprolactone and a collagen/poly-ε-caprolactone blend. Biomaterials 2007, 28, 3012–3025. [Google Scholar] [CrossRef]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef]
- Ma, Z.; He, W.; Yong, T.; Ramakrishna, S. Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Eng. 2005, 11, 1149–1158. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Carmagnola, I.; Gentile, P.; Boccafoschi, F.; Tonda-Turo, C.; Ballarini, M.; Georgieva, V.; Georgiev, G.; Ciardelli, G. Layer-by-layer coating of photoactive polymers for biomedical applications. Surf. Coatings Technol. 2012, 206, 2446–2453. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Boccafoschi, F.; Baino, F.; Vitale-Brovarone, C.; Vernè, E.; Barbani, N.; Ciardelli, G. Composite Films of Gelatin and Hydroxyapatite/Bioactive Glass for Tissue-Engineering Applications. J. Biomater. Sci. Polym. Ed. 2010, 21, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, Y.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Meng, Z.; Xu, X.; Zheng, W.; Zhou, H.; Li, L.; Zheng, Y.; Lou, X. Preparation and characterization of electrospun PLGA/gelatin nanofibers as a potential drug delivery system. Colloids Surfaces B Biointerfaces 2011, 84, 97–102. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.-B.; Chen, W.-T.; Chien, H.-W.; Kuo, W.-H.; Wang, M.-J. Poly(dopamine) coating of scaffolds for articular cartilage tissue engineering. Acta Biomater. 2011, 7, 4187–4194. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Zhao, W.; Wei, Q.; Nie, S.; Sun, S.; Zhao, C. The hydrodynamic permeability and surface property of polyethersulfone ultrafiltration membranes with mussel-inspired polydopamine coatings. J. Membr. Sci. 2012, 417–418, 228–236. [Google Scholar] [CrossRef]
- Rim, N.G.; Kim, S.J.; Shin, Y.M.; Jun, I.; Lim, N.W.; Park, J.H.; Shin, H. Mussel-inspired surface modification of poly(l-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surfaces B Biointerfaces 2012, 91, 189–197. [Google Scholar] [CrossRef]
- Ku, S.H.; Park, C.B. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef]
- Burzio, L.A.; Waite, J.H. Cross-Linking in Adhesive Quinoproteins: Studies with Model Decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lee, J.S.; Kim, J.; Bin Lee, Y.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.-W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.-H.; Cho, H.-J.; Kim, H.K.; Yoon, T.-R.; Shin, H. Electrospun fibers immobilized with bone forming peptide-1 derived from BMP7 for guided bone regeneration. Biomaterials 2013, 34, 5059–5069. [Google Scholar] [CrossRef]
- Xie, J.; Michael, P.L.; Zhong, S.; Ma, B.; MacEwan, M.R.; Lim, C.T. Mussel inspired protein-mediated surface modification to electrospun fibers and their potential biomedical applications. J. Biomed. Mater. Res. Part A 2012, 100, 929–938. [Google Scholar] [CrossRef]
- Zhao, J.; Han, F.; Zhang, W.; Yang, Y.; You, D.; Li, L. Toward improved wound dressings: Effects of polydopamine-decorated poly(lactic-co-glycolic acid) electrospinning incorporating basic fibroblast growth factor and ponericin G1. RSC Adv. 2019, 9, 33038–33051. [Google Scholar] [CrossRef] [Green Version]
- Nardo, T.; Chiono, V.; Ciardelli, G.; Tabrizian, M. PolyDOPA Mussel-Inspired Coating as a Means for Hydroxyapatite Entrapment on Polytetrafluoroethylene Surface for Application in Periodontal Diseases. Macromol. Biosci. 2015, 16, 288–298. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wäigung diinner Schichten und zur Mikrowäigung (Use of quartz crystals for weighing thin layers and for microweighing). Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Wang, Y.; Zhu, B.-K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y.; Wang, Y.; Zhang, L.; Wang, W. Preparation and characterization of dopamine-decorated hydrophilic carbon black. Appl. Surf. Sci. 2012, 258, 5387–5393. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M.; Pauvert, B.; Terol, A. Vibrational analysis of poly(L-lactic acid). J. Raman Spectrosc. 1995, 26, 307–311. [Google Scholar] [CrossRef]
- Smith, P.B.; Leugers, A.; Kang, S.; Hsu, S.L.; Yang, X. An analysis of the correlation between structural anisotropy and dimensional stability for drawn poly(lactic acid) films. J. Appl. Polym. Sci. 2001, 82, 2497–2505. [Google Scholar] [CrossRef]
- Ye, W.; Wang, D.; Zhang, H.; Zhou, F.; Liu, W. Electrochemical growth of flowerlike gold nanoparticles on polydopamine modified ITO glass for SERS application. Electrochim. Acta 2010, 55, 2004–2009. [Google Scholar] [CrossRef]
- Yang, X.; Kang, S.; Yang, Y.; Aou, K.; Hsu, S.L. Raman spectroscopic study of conformational changes in the amorphous phase of poly(lactic acid) during deformation. Polymer 2004, 45, 4241–4248. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.; Muzio, G.; Canuto, R.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.M.; Shoichet, M.S.; Davies, J.E. Bone formation on two-dimensional poly(DL-lactide-co-glycolide) (PLGA) films and three-dimensional PLGA tissue engineering scaffoldsin vitro. J. Biomed. Mater. Res. 2003, 64, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Das, T.K.; Saha, S. Synthesis and characterization of CdS nanoparticles. J. Mater. Sci. Mater. Electron. 2011, 22, 1761–1765. [Google Scholar] [CrossRef]
- Park, H.H.; Lee, K.Y.; Lee, S.J.; Park, K.E.; Park, W.H. Plasma-treated poly(lactic-co-glycolic acid) nanofibers for tissue engineering. Macromol. Res. 2007, 15, 238–243. [Google Scholar] [CrossRef]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-Step Modification of Superhydrophobic Surfaces by a Mussel-Inspired Polymer Coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Tonda-Turo, C.; Carmagnola, I.; Ciardelli, G. Quartz Crystal Microbalance with Dissipation Monitoring: A Powerful Method to Predict the in vivo Behavior of Bioengineered Surfaces. Front. Bioeng. Biotechnol. 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Bin Lee, Y.; Kim, S.J.; Kang, J.K.; Park, J.-C.; Jang, W.; Shin, H. Mussel-Inspired Immobilization of Vascular Endothelial Growth Factor (VEGF) for Enhanced Endothelialization of Vascular Grafts. Biomacromolecules 2012, 13, 2020–2028. [Google Scholar] [CrossRef]
- Wei, Q.; Li, B.; Yi, N.; Su, B.; Yin, Z.; Zhang, F.; Li, J.; Zhao, C. Improving the blood compatibility of material surfaces via biomolecule-immobilized mussel-inspired coatings. J. Biomed. Mater. Res. Part A 2010, 96, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ryou, M.-H.; Lee, Y.M.; Park, J.-K.; Choi, J.W. Mussel-Inspired Polydopamine-Treated Polyethylene Separators for High-Power Li-Ion Batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Gentile, P.; Ciardelli, G.; Cannillo, V. Biomimetic coating on bioactive glass-derived scaffolds mimicking bone tissue. J. Biomed. Mater. Res. Part A 2012, 100, 3259–3266. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, G.; Huangfu, X.; Zhao, J.; Bohu, Y.; Klouche, S.; Lefevre, N.; Gerometta, A. Gelatin-Grafted Electrospun Fibrous Membranes for Rotator Cuff Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, e56–e57. [Google Scholar] [CrossRef]









| Tg (°C) | Mn (g/mol) | Mw (g/mol) | Mz (g/mol) | Polydispersity Index (PDI) | |
|---|---|---|---|---|---|
| PLGA | 48.9 | 40,700 | 68,600 | 96,600 | 1.69 |
| PLGA-DOPA | 49.2 | 46,900 | 72,000 | 98,500 | 1.54 |
| Abs | mol/L (×10−5) | nmol/cm2 | |
|---|---|---|---|
| PLGA | 0.05 ± 0.01 | 4.97 ± 0.53 | 2.49 ± 0.26 |
| PLGA-DOPA | 0.06 ± 0.02 | 5.84 ± 2.32 | 29.2 ± 11.60 |
| PLGA-G | 0.05 ± 0.01 | 5.28 ± 0.65 | 26.4 ± 3.27 |
| PLGA-DOPA-G | 0.12 ± 0.01 | 11.8 ± 1.16 | 58.9 ± 5.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmagnola, I.; Chiono, V.; Ruocco, G.; Scalzone, A.; Gentile, P.; Taddei, P.; Ciardelli, G. PLGA Membranes Functionalized with Gelatin through Biomimetic Mussel-Inspired Strategy. Nanomaterials 2020, 10, 2184. https://doi.org/10.3390/nano10112184
Carmagnola I, Chiono V, Ruocco G, Scalzone A, Gentile P, Taddei P, Ciardelli G. PLGA Membranes Functionalized with Gelatin through Biomimetic Mussel-Inspired Strategy. Nanomaterials. 2020; 10(11):2184. https://doi.org/10.3390/nano10112184
Chicago/Turabian StyleCarmagnola, Irene, Valeria Chiono, Gerardina Ruocco, Annachiara Scalzone, Piergiorgio Gentile, Paola Taddei, and Gianluca Ciardelli. 2020. "PLGA Membranes Functionalized with Gelatin through Biomimetic Mussel-Inspired Strategy" Nanomaterials 10, no. 11: 2184. https://doi.org/10.3390/nano10112184