Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
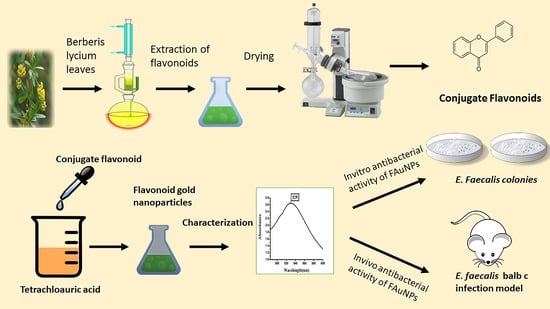
2.2. Flavonoid Extraction
2.3. Green Synthesis of FAuNPs
2.4. Physical Characterizations of FAuNPs
2.5. In-Vitro Stability of FAuNPs
2.6. Antibacterial Susceptibility of Flavonoids by Qualitative Method
2.7. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of CF and FAuNPs Against E. faecalis
2.8. Hemolysis Assay
2.9. Colonization of E. faecalis in BALB/c Mice
2.10. In Vivo Antibacterial Activity of CF and FAuNPs
2.11. Statistical Analysis
3. Results and Discussion
3.1. Antimicrobial Activity of Flavonoids
3.2. Synthesis and Physical Characterizations of FAuNPs
3.2.1. Impact of Physico-Chemical Parameters on FAuNPs Synthesis and Its Stability
3.2.2. FTIR Analysis of CF and FAuNPs
3.2.3. XRD Analysis of FAuNPs
3.2.4. STEM Analysis of FAuNPs
3.2.5. EDS Analysis (EDAX) of FAuNPs
3.3. In Vitro Antibacterial Activity of FAuNPs
3.4. Hemolysis Assay
3.5. In Vivo Anticolonizing Potential of CF and FAuNPs in Infectious Mice Model
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| B. lyceum: | Berberis lycium |
| C.E: | Crude extract |
| C.F: | Conjugated flavonoids |
| E. faecalis: | Enterococcus faecalis |
| EDS: | Energy-dispersive X-ray spectroscopy |
| FAuNPs: | Flavonoid gold nanoparticles |
| F.F: | Free flavonoids |
| FTIR: | Fourier-transform infrared spectroscopy |
| HAuCl4: | Tetrachloroauric acid |
| IV: | Intravenous |
| MDR: | Multi-drug resistance |
| NPs: | Nanoparticles |
| RBCs: | Red blood cells |
| STEM: | Scanning transmission electron microscopy |
| UTI: | Urinary tract infection |
| UV-Vis: | Ultraviolet-visible |
References
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Menaa, F. When Pharma Meets Nano or The Emerging Era of Nano-Pharmaceuticals. Pharm. Anal. Acta 2013, 4, 223. [Google Scholar] [CrossRef] [Green Version]
- Loomba, L.; Scarabelli, T. Metallic nanoparticles, and their medicinal potential. Part I: Gold and silver colloids. Ther. Deliv. 2013, 4, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ho, P.; Tong, E.; Wang, L.; Xu, B. Presenting Vancomycin on Nanoparticles to Enhance Antimicrobial Activities. Nano Lett. 2003, 3, 1261–1263. [Google Scholar] [CrossRef]
- Rosemary, M.; MacLaren, I.; Pradeep, T. Investigations of the Antibacterial Properties of Ciprofloxacin@SiO2. Langmuir 2006, 22, 10125–10129. [Google Scholar] [CrossRef]
- Selvaraj, V.; Alagar, M. Analytical detection and biological assay of antileukemic drug 5-fluorouracil using gold nanoparticles as probe. Int. J. Pharm. 2007, 337, 275–281. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sahu, S.; Pramanik, P.; Roy, S. In vitro antimicrobial activity of nanoconjugated vancomycin against drug resistant Staphylococcus aureus. Int. J. Pharm. 2012, 436, 659–676. [Google Scholar] [CrossRef]
- Grace, N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles a brief study. Colloids and Surfaces A: Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Grace, N.; Pandian, K. Quinolone antibiotic-capped gold nanoparticles and their antibacterial efficacy against gram positive and gram-negative organisms. J. Bionanosci. 2007, 1, 96–105. [Google Scholar] [CrossRef]
- Brown, A.; Smith, K.; Samuels, T.; Lu, J.; Obare, S.; Scott, M. Nanoparticles Functionalized with Ampicillin Destroy Multiple-Antibiotic-Resistant Isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and Methicillin-Resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef] [Green Version]
- Chamundeeswari, M.; Sobhana, S.; Jacob, J.; Kumar, M.; Devi, M.; Sastry, T.; Mandal, A. Preparation, characterization and evaluation of a biopolymeric gold nanocomposite with antimicrobial activity. Biotechnol. Appl. Biochem. 2010, 55, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Varisco, M.; Khanna, N.; Brunetto, P.; Fromm, K. New Antimicrobial and Biocompatible Implant Coating with Synergic Silver-Vancomycin Conjugate Action. ChemMedChem 2014, 9, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, J.; Chen, W.; Luo, L.; Wei-Guang Diau, E.; Chen, Y. Functional gold nanoclusters as antimicrobial agents for antibiotic-resistant bacteria. Nanomedicine 2010, 5, 755–764. [Google Scholar] [CrossRef]
- Rana, N.; Sauvageot, N.; Laplace, J.; Bao, Y.; Nes, I.; Rincé, A.; Posteraro, B.; Sanguinetti, M.; Hartke, A. Redox Balance via Lactate Dehydrogenase Is Important for Multiple Stress Resistance and Virulence in Enterococcus faecalis. Infect. Immun. 2013, 81, 2662–2668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, C.; Contreras, G.; Murray, B. Management of multidrug-resistant enterococcal infections. Clin. Microbiol. Infect. 2010, 16, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Michaux, C.; Sanguinetti, M.; Reffuveille, F.; Auffray, Y.; Posteraro, B.; Gilmore, M.; Hartke, A.; Giard, J. SlyA Is a Transcriptional Regulator Involved in the Virulence of Enterococcus faecalis. Infect. Immun. 2011, 79, 2638–2645. [Google Scholar] [CrossRef] [Green Version]
- Gentry-Weeks, C.; Estay, M.; Loui, C.; Baker, D. Intravenous Mouse Infection Model for Studying the Pathology of Enterococcus faecalis Infections. Infect. Immun. 2003, 71, 1434–1441. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.; Webber, A.; Baylay, J.; Ogbolu, O.; Piddock, J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Mohammed Fayaz, A.; Girilal, M.; Mahdy, S.; Somsundar, S.; Venkatesan, R.; Kalaichelvan, P. Vancomycin bound biogenic gold nanoparticles: A different perspective for development of anti VRSA agents. Process Biochem. 2011, 46, 636–641. [Google Scholar] [CrossRef]
- Dianat, O.; Saedi, S.; Kazem, M.; Alam, M. Antimicrobial Activity of Nanoparticle Calcium Hydroxide against Enterococcus Faecalis: An In vitro study. Iran Endod. J. 2014, 10, 39–43. [Google Scholar]
- Barreras, U.; Méndez, F.; Martínez, R.; Valencia, C.; Rodríguez, P.; Rodríguez, J. Chitosan nanoparticles enhance the antibacterial activity of chlorhexidine in collagen membranes used for periapical guided tissue regeneration. Mater. Sci. Eng. C 2016, 58, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Fan, B. Substantivity of Ag–Ca–Si mesoporous nanoparticles on dentin and its ability to inhibit Enterococcus faecalis. J. Mater. Sci. Mater. Med. 2015, 27. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus faecalis Biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Prodan, A.M.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Synthesis and characterization of polysaccharide-maghemite composite nanoparticles and their antibacterial properties. Nanoscale Res. Lett. 2012, 7, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, P.; Nayak, V.; Kowshik, M. Role of gold nanoparticles as drug delivery vehicles for chondroitin sulfate in the treatment of osteoarthritis. Biotechnol. Prog. 2015, 31, 1416–1422. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Rajesh, S. Bionanoparticles: Synthesis and antimicrobial applications. In Science against microbial pathogens: Communicating current research and technological advances. Badajoz Spain 2011, 23, 228–244. [Google Scholar]
- Ehrenberg, M.S.; Friedman, A.E.; Finkelstein, J.N.; Oberdörster, G.; Mcgrath, J.L. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomaterials 2009, 30, 603–610. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; Mcneil, S.E. Preclinical Studies To Understand Nanoparticle Interaction with the Immune System and Its Potential Effects on Nanoparticle Biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- . El-Azizi, M.M.; Din, S.N.E.; El-Tayeb, T.; Aisha, K.A. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int. J. Nanomed. 2016, 11, 1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.; Li, N.; Zhang, W.; Yang, E.; Mou, Z.; Zhao, Z.; Liu, H.; Wang, W. Quercetin-loaded PLGA nanoparticles: A highly effective antibacterial agent in vitro and anti-infection application in vivo. J. Nanoparticle Res. 2015, 18, 3. [Google Scholar] [CrossRef]
- Cochrane, S.A.; Li, X.; He, S.; Yu, M.; Wu, M.; Vederas, J.C. Synthesis of Tridecaptin–Antibiotic Conjugates with in Vivo Activity against Gram-Negative Bacteria. J. Med. Chem. 2015, 58, 9779–9785. [Google Scholar] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Ateeq, M.; Shah, M.R.; Ain, N.U.; Bano, S.; Anis, I.; Anis, I.; Lubna; Faizi, S.; Bertino, M.F.; Naz, S.S. Green synthesis and molecular recognition ability of patuletin coated gold nanoparticles. Biosens. Bioelectron. 2015, 63, 499–505. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2018, 33, 13–40. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Uddin, S.; Jalal, S. Chemistry and Biological Activities of Berberis lyceum Royle. J. Biol. Act. Prod. Nat. 2015, 5, 295–312. [Google Scholar]
- Jacob, A.E.; Hobbs, S.J. Conjugal Transfer of Plasmid-Borne Multiple Antibiotic Resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 1974, 117, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P.; Kulshrestha, N.; Rafiee, J.; Koratkar, N.; Misra, D.S. Graphene Supported Platinum Nanoparticle Counter-Electrode for Enhanced Performance of Dye-Sensitized Solar Cells. Acs Appl. Mater. Interfaces 2011, 3, 3884–3889. [Google Scholar] [CrossRef]
- Mir, M.A.; Sawhney, S.S.; Jassal, M.M.S. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharmocology 2013, 2, 01–05. [Google Scholar]
- Inalegwu, B.; Sodipo, O. Phytochemical screening and haemolytic activities of crude and purified saponins of aqueous and methanolic extracts of leaves of Tephrosia vogelii hook. F. Asian J. Plant Sci. Res. 2013, 3, 7–11. [Google Scholar]
- Naz, S.S.; Islam, N.U.; Shah, M.R.; Alam, S.S.; Iqbal, Z.; Bertino, M.; Franzel, L.; Ahmed, A. Enhanced biocidal activity of Au nanoparticles synthesized in one pot using 2, 4-dihydroxybenzene carbodithioic acid as a reducing and stabilizing agent. J. Nanobiotechnology 2013, 11, 13. [Google Scholar] [CrossRef] [Green Version]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Path. 1996, 45, 493–496. [Google Scholar] [CrossRef]
- Muhammad, Z.; Raza, A.; Ghafoor, S.; Naeem, A.; Naz, S.S.; Riaz, S.; Ahmed, W.; Rana, N.F. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur. J. Pharm. Sci. 2016, 91, 251–255. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Akinleye, T.E.; Segun, P.A.; Faleye, T.C.; Adeniji, A.J. In vitro antiviral activity of twenty-seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. Virol. J. 2018, 15, 110. [Google Scholar] [CrossRef]
- Jaime, M.F.V.; Redko, F.; Muschietti, L.V.; Campos, R.H.; Martino, V.S.; Cavallaro, L.V. In vitro antiviral activity of plant extracts from Asteraceae medicinal plants. Virol. J. 2013, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-H.; Zhang, X.; Choi, Y.-J.; Park, H.-H.; Hill, R.H. Synthesis of Ag Nanostructures by Photochemical Reduction Using Citrate-Capped Pt Seeds. J. Nanomater. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar. P. Production, isolation and identification of flavonoid from aerial parts of hiptage benghalensis. Int. J. Life Sci. Pharm. Res. 2012, 2, 1–5. [Google Scholar]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids Loaded in Nanocarriers: An Opportunity to Increase Oral Bioavailability and Bioefficacy. Food Nutr. Sci. 2014, 05, 1212–1327. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An Evaluation of Blood Compatibility of Silver Nanoparticles. Sci. Rep. 2016, 6, 1–15. [Google Scholar]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, S.; Fatima Rana, N.; Hussain, I.; Tanweer, T.; Nawaz, A.; Menaa, F.; Janjua, H.A.; Alam, T.; Batool, A.; Naeem, A.; et al. Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs. Nanomaterials 2020, 10, 1769. https://doi.org/10.3390/nano10091769
Riaz S, Fatima Rana N, Hussain I, Tanweer T, Nawaz A, Menaa F, Janjua HA, Alam T, Batool A, Naeem A, et al. Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs. Nanomaterials. 2020; 10(9):1769. https://doi.org/10.3390/nano10091769
Chicago/Turabian StyleRiaz, Sundus, Nosheen Fatima Rana, Irshad Hussain, Tahreem Tanweer, Afrah Nawaz, Farid Menaa, Hussnain A. Janjua, Tahseen Alam, Amna Batool, Ayesha Naeem, and et al. 2020. "Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs" Nanomaterials 10, no. 9: 1769. https://doi.org/10.3390/nano10091769