Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials
Abstract
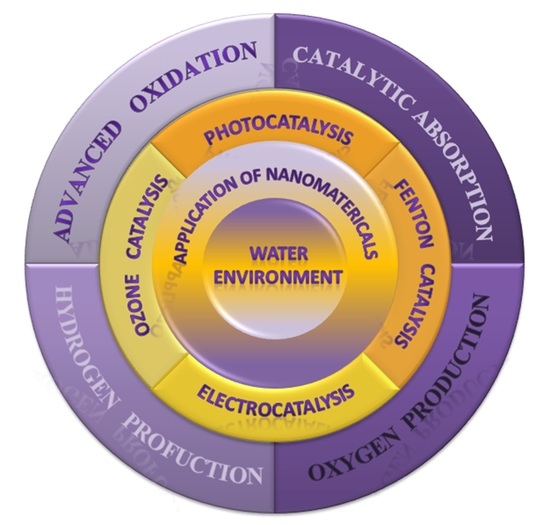
:1. Introduction
2. Classification of Nanomaterial Catalysts in Water
2.1. Research Background and Significance of Nanomaterials
2.2. The Basic Composition of Nanocatalyst
2.3. Nano-Metal Material Monomer and Compounds
2.3.1. Nano-Metal Catalyst
2.3.2. Nanometallic Oxides and Metal Sulfides
2.3.3. Nano-Metallic Hydrogen Oxides
2.3.4. Nanocarbon Related Materials (Carbon Nanotubes, Carbon Nano Types, Carbon Nanometers, Carbon Quantum Points)
2.4. Nanometallic Organic Skeleton MOFs and Other Materials
2.5. Research Problems and Directions of Nanomaterials
3. Advanced Oxidation
3.1. Advanced Oxidation Research Status and Content
3.2. Photochemical Oxidation
3.3. Electrochemical Oxidation
3.4. Fenton Oxidation
3.5. Ozone Oxidation
4. Adsorption Catalysis
4.1. Heavy Metal Wastewater Treatment Status and Solutions
- ①.
- Due to the effect of ultrasound, interconnected nanoparticles with irregular shapes are distributed on the film layer, and the average diameter of these nanoparticles is about 100 nm, as shown in Figure 6A.
- ②.
- As a result of the protein carbonization process, these nanoparticles (about 3–5 nm) are distributed on the surface of the film (carbonized ESM) (Figure 6B).
- ③.
- In addition, these small nanoparticles are identified as CuO or ZnO nanoparticles. The corresponding HR-TEM image in Figure 6C shows discontinuous lattice fringes. These results indicate that CZ-ESM is a heterogeneous nanocomposite material.
- ①.
- ②.
- Figure 6C shows the effective catalytic activity of the synthesized nanocomposite, which reduces to 4-AP after 12 min. The kinetic constant (k) is also calculated by using the pseudo-first-order kinetic model (dC/dt = kC). The relationship diagram in Figure 7D between ln(C/C0) and t confirms that the catalytic system follows the pseudo-first-order kinetic model, and the k value of CZ-ESM is 0.7919 min−1. The results show that the CZ-ESM nanocomposite shows significant activity and can be used as an effective catalyst for 4-NP reduction.
4.2. Existing Problems and Development of Nano-Sorption
5. Hydrolysis Catalysis (Hydrogen Analysis Reaction and Oxygen Analysis Reaction)
5.1. Photocatalysis
- (1)
- Pair can be retouched:
- (2)
- Improvement of crystal type
- (3)
- Light sensitization
5.2. Electric Catalysis
6. The Current Status of Nano Research
7. Existing Research Problems and Future Research Directions
- (1)
- The flow of nanomaterials into the environment may have a profound impact on living things. Nanomaterials will inevitably flow into the environment during production, use, and disposal [20,21]. The activated or generated active oxygen clusters, such as the hydroxyl groups often mentioned above, will cause significant oxidative damage to biological cells. Dissolution will permanently exist in the environment and cause poisoning to organisms. To reduce the ecological and environmental risks promptly, it is necessary to improve the chemical stability of the nanomaterials, extend their cycle, or magnetically separate the metal nanomaterials to reduce the difficulties caused by filtration and centrifugal separation during the recovery process. On the other hand, completing the biosafety evaluation system should not be delayed. The use of uncontrollable nanomaterials for the long-term in the environment should be avoided [33].
- (2)
- The limitation of the light absorption range of nanomaterials. At present, high-performance nanocatalysts generally have a wider bandgap and focus on the absorption of ultraviolet light, thereby greatly reducing the utilization rate of visible light and limiting large-scale industrial applications. Finding a more efficient and reliable light source is a way to improve the photocatalytic efficiency, such as amalgam lamps [59], diodes, etc., which is a problem that the research community needs to focus on to find efficient light sources.
- (3)
- Development and utilization of other hydrogen storage energy. The development of low-cost hydrogen production capacity and efficient water electrolysis route is very attractive. However, it requires too much energy to overcome the activation energy of the anode side oxygen release reaction. Therefore, chemical hydrogen storage materials with high hydrogen storage capacity and easy dehydrogenation have also attracted people’s attention, such as hydrazine hydrate and ammonia boron [74], hydrocarbons (formic acid, methanol [70], etc.), and urea [75]. Such as non-toxic and flammable high-efficiency hydrogen storage materials, which can produce more hydrogen than water in a short time at room temperature. The development of catalysts that can promote the production of hydrogen from these hydrogen storage materials is the future development and utilization of water removal potential strategic goals for other hydrogen storage materials [6].
- (4)
- Nanomaterials themselves are corroded in the environment, resulting in short service life. To achieve the long-term use of nanomaterials, the circulation of active ingredients flowing into the environment and the development of corrosion-resistant materials are particularly important development directives for regenerating nanomaterials [80].
8. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russell, D.L. What is Water Pollution? Practical Wastewater Treatment, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mao, S.S.; Shen, S.; Guo, L. Nanomaterials for renewable hydrogen production, storage and utilization. Prog. Nat. Sci. 2012, 22, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Shaik, A.A.N.N. Nanomaterials: Solutions to water-concomitant challenges. Membranes 2019, 9, 40. [Google Scholar]
- Aquaculture, R.U. McNevin, water pollution. In The Environment, 1st ed.; Claude, E., Boyd, A.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; p. 211. [Google Scholar]
- Schell, L.M. Modern water: A biocultural approach to water pollution at the Akwesasne Mohawk Nation. Am. J. Hum. Boil. 2020, 32, e23348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J. Recent progress in graphene-based noble-metal nanocomposites for electrocatalytic applications. Adv. Mater. 2019, 31, e1800696. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, S.K. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192. [Google Scholar]
- Deng, F. Nanomaterial-based photocatalytic hydrogen production. In Nanomaterials for the Removal of Pollutants and Resource Reutilization, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–82. [Google Scholar]
- Chen, X. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef]
- Bayan, E.M. Zn–F co-doped TiO2 nanomaterials: Synthesis, structure and photocatalytic activity. J. Alloy. Compd. 2020, 822. [Google Scholar] [CrossRef]
- Niu, L. Ce-based catalysts used in advanced oxidation processes for organic wastewater treatment: A review. J. Environ. Sci. 2020, 96, 109–116. [Google Scholar] [CrossRef]
- Yuan, J. MOF derived ZnSe–FeSe2/RGO Nanocomposites with enhanced sodium/potassium storage. J. Power Sources 2020, 455, 227937. [Google Scholar] [CrossRef]
- Xiao, Y. CuGeO3 micro-nanomaterial as Electrocatalyst for hydrogen evolution reaction. Catal. Commun. 2020, 144, 106075. [Google Scholar] [CrossRef]
- Radecka, M. Oxide Nanomaterials for photoelectrochemical hydrogen energy sources, in materials for sustainable energy. Co2 Chem. 2018, 72, 145–183. [Google Scholar]
- Wang, T.; Zhang, S. Binary NiCu layered double hydroxide nanosheets for enhanced energy storage performance as supercapacitor electrode. Sci. China Mater. 2017, 61, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Alammari, W.; Govindhan, M.; Chen, A. Modification of TiO2 Nanotubes with PtRu/Graphene Nanocomposites for Enhanced Oxygen Reduction Reaction. ChemElectroChem 2015, 2, 2041–2047. [Google Scholar] [CrossRef]
- Soo, J.Z. Optimal electrospun TiO2 nanofiber photocatalytic performance via synergistic morphology and particle crystallinity with anatase/rutile phase tuning. Phys. Status Solidi 2019, 216, 1900066. [Google Scholar] [CrossRef]
- Aravindan, V. Unveiling TiNb2O7 as an insertion anode for lithium ion capacitors with high energy and power density. ChemSusChem 2014, 7, 1858–1863. [Google Scholar] [CrossRef]
- Gandavadi, D.; Sundarrajan, S.; Ramakrishna, S. Bio-based nanofibers involved in wastewater treatment. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Li, P.; Zeng, H.C. Sandwich-Like Nanocomposite of CoNiOx/Reduced graphene oxide for enhanced electrocatalytic water oxidation. Adv. Funct. Mater. 2017, 27, 1606325. [Google Scholar] [CrossRef]
- Nidheesh, P.; Babu, D.S.; Dasgupta, B.; Behara, P.; Ramaswamy, B.; Kumar, M.S. Treatment of arsenite-contaminated water by electrochemical advanced oxidation processes. ChemElectroChem 2020, 7, 2418–2423. [Google Scholar] [CrossRef]
- Tahir, A.A.; Ullah, H.; Sudhagar, P.; Teridi, M.A.M.; Devadoss, A.; Sundaram, S. ChemInform abstract: The application of graphene and its derivatives to energy conversion, storage, and environmental and biosensing devices. Chem. Rec. 2016, 47, 1591–1634. [Google Scholar] [CrossRef]
- Pattanayak, P.; Pramanik, N.; Papiya, F.; Kumar, V.; Kundu, P.P. Metal-free keratin modified poly(pyrrole-co-aniline)-reduced graphene oxide based nanocomposite materials: A promising cathode catalyst in microbial fuel cell application. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Malgras, V.; Na, J.; Lin, J.; You, J.; Zhang, M.; Li, J.; Yamauchi, Y. Metal-organic frameworks and their derived materials: Emerging catalysts for a sulfate radicals-based advanced oxidation process in water purification. Small 2019, 15, e1900744. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-doped carbon nanomaterials: To the mechanism of growth, electrical conductivity and application in catalysis. Catal. Today 2015, 249, 12–22. [Google Scholar] [CrossRef]
- Fu, M.; Liu, Y.; Zhang, Q.; Ning, G.; Fan, X.; Wang, H.; Lu, H.; Zhang, Y.; Wang, H. Fe2O3 and Co bimetallic decorated nitrogen doped graphene nanomaterial for effective electrochemical water split hydrogen evolution reaction. J. Electroanal. Chem. 2019, 849. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, R. Multifunctional nanostructured electrocatalysts for energy conversion and storage: Current status and perspectives. Nanoscale 2018, 10, 11241–11280. [Google Scholar] [CrossRef]
- Toyama, H.; Bessho, K.; Huang, L.; Hirota, S.K.; Kano, Y.; Mase, K.; Sato, T.; Naiki, A.; Li, J.; Shimatani, Y.; et al. The effects of water pollution on the phylogenetic community structure of aquatic plants in the East Tiaoxi River, China. Freshw. Boil. 2019, 65, 632–645. [Google Scholar] [CrossRef]
- Jin, R.; Qiu, Z.; Cheng, W.; Jin, X. Photocatalytic degradation of aniline by magnetic nanomaterials Fe3O4@SiO2@BiO1.8·0.04H2O/Ag3PO4. Chem. Phys. Lett. 2020, 137747. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Nunes, M.; Fernandes, D.M.; Rocha, I.M.; Pereira, M.F.R.; Mbomekalle, I.M.; De Oliveira, P.; Freire, C. Phosphomolybdate@Carbon-Based Nanocomposites as Electrocatalysts for Oxygen Reduction Reaction. ChemistrySelect 2016, 1, 6257–6266. [Google Scholar] [CrossRef]
- Khan, W.U.; Fakeeha, A.H.; Al-Fatesh, A.S.; Ibrahim, A.A.; Abasaeed, A.E. La2O3 supported bimetallic catalysts for the production of hydrogen and carbon nanomaterials from methane. Int. J. Hydrog. Energy 2016, 41, 976–983. [Google Scholar] [CrossRef]
- Cao, C.; Ma, D.-D.; Xu, Q.; Wu, X.-T.; Zhu, Q.-L. Semisacrificial Template Growth of Self-Supporting MOF Nanocomposite Electrode for Efficient Electrocatalytic Water Oxidation. Adv. Funct. Mater. 2018, 29. [Google Scholar] [CrossRef]
- Sadoun, A.; Mohammed, M.; Elsayed, E.; Meselhy, A.; El-Kady, O.A. Effect of nano Al2O3 coated Ag addition on the corrosion resistance and electrochemical behavior of Cu-Al2O3 nanocomposites. J. Mater. Res. Technol. 2020, 9, 4485–4493. [Google Scholar] [CrossRef]
- Mahmoud, H.R.; El-Molla, S.A.; Naghmash, M.A. Novel mesoporous MnO2/SnO2 nanomaterials synthesized by ultrasonic-assisted co-precipitation method and their application in the catalytic decomposition of hydrogen peroxide. Ultrasonics 2019, 95, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W.; Al-Sayari, S.A. Synthesis and photocatalytic properties of nanocrystalline Au, Pd and Pt photodeposited onto mesoporous RuO2-TiO2 nanocomposites. Appl. Catal. A Gen. 2012, 431, 62–68. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, G.; Ye, L. Preparation and adsorption mechanism of polyvinyl alcohol/graphene oxide-sodium alginate nanocomposite hydrogel with high Pb(II) adsorption capacity. J. Appl. Polym. Sci. 2018, 136. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H. Nanocomposites Consisting of Silver Sulfide and Noble Metals; Springer: Cham, Switzerland, 2014; pp. 93–113. [Google Scholar]
- Wang, J.; Lim, Y.-F.; Ho, G.W. Manganese copper sulfide nanocomposites: Structure tailoring and photo/electrocatalytic hydrogen generation. ChemCatChem 2017, 9, 4148–4154. [Google Scholar] [CrossRef]
- Di, J.; Zhu, M.; Jamakanga, R.; Gai, X.; Li, Y.; Yang, R. Electrochemical activation combined with advanced oxidation on NiCo2O4 nanoarray electrode for decomposition of Rhodamine B. J. Water Process. Eng. 2020, 37, 101386. [Google Scholar] [CrossRef]
- Goswami, M.; Ghosh, R.; Maruyama, T.; Meikap, A.K. Polyaniline/carbon nanotube/CdS quantum dot composites with enhanced optical and electrical properties. Appl. Surf. Sci. 2016, 364, 176–180. [Google Scholar] [CrossRef]
- Savadkoohi, M.; Dorranian, D.; Solati, E. Using silicon nanoparticles to modify the surface of graphene nanosheets. Mater. Sci. Semicond. Process. 2018, 75, 75–83. [Google Scholar] [CrossRef]
- Zhang, H.; Ying, M.; Gao, R.; Hu, L.; Jiao, Z.; Zhu, X. Carbon-mediated fabrication of core–shell structured SnO2@TiO2 nanocomposites with excellent photocatalytic performance. RSC Adv. 2015, 5, 58439–58448. [Google Scholar] [CrossRef]
- Farhadi, S.; Mahmoudi, F. Improving the adsorption ability of perovskite-type LaNiO3 nanomaterial towards organic dyes by hybridizing with phosphotungstic acid. Polyhedron 2019, 169, 39–50. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Sun, Y.; Chen, Y.; Chen, H.; Han, S.; Lin, H. Fe2O3 nanocatalysts on N-doped carbon nanomaterial for highly efficient electrochemical hydrogen evolution in alkaline. J. Power Sources 2019, 426, 74–83. [Google Scholar] [CrossRef]
- Zhan, F.; Yin, J.; Zhang, A.; Zhou, J.; Wang, M.; Jiao, T. Controllable morphology and highly efficient catalytic performances of Pd–Cu bimetallic nanomaterials prepared via seed-mediated co-reduction synthesis. Appl. Surf. Sci. 2020, 527, 146719. [Google Scholar] [CrossRef]
- Suanon, F.; Tang, L.; Sheng, H.; Fu, Y.; Xiang, L.; Wang, Z.; Shao, X.; Mama, D.; Jiang, X.; Wang, F. Organochlorine pesticides contaminated soil decontamination using TritonX-100-enhanced advanced oxidation under electrokinetic remediation. J. Hazard. Mater. 2020, 393, 122388. [Google Scholar] [CrossRef]
- Naeem, H.; Ajmal, M.; Qureshi, R.B.; Muntha, S.T.; Farooq, M.; Siddiq, M. Facile synthesis of graphene oxide-silver nanocomposite for decontamination of water from multiple pollutants by adsorption, catalysis and antibacterial activity. J. Environ. Manag. 2018, 230, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Samadi, M.; Sarikhani, N.; Zirak, M.; Zhang, H.; Zhang, H.-L.; Moshfegh, A.Z. Group 6 transition metal dichalcogenide nanomaterials: Synthesis, applications and future perspectives. Nanoscale Horiz. 2018, 3, 90–204. [Google Scholar] [CrossRef]
- Sarwar, S. Facile microwave approach towards high performance MoS2/graphene nanocomposite for hydrogen evolution reaction. Sci. China Mater. 2019, 63, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Nithiyanantham, U.; Ramadoss, A.; Kundu, S. Supercapacitor and dye-sensitized solar cell (DSSC) applications of shape-selective TiO2 nanostructures. RSC Adv. 2014, 4, 35659–35672. [Google Scholar] [CrossRef]
- Zhang, T.; Lowry, G.V.; Cápiro, N.L.; Chen, J.; Chen, W.; Chen, Y.; Dionysiou, D.D.; Elliott, D.W.; Ghoshal, S.; Hofmann, T.; et al. In situ remediation of subsurface contamination: Opportunities and challenges for nanotechnology and advanced materials. Environ. Sci. Nano 2019, 6, 1283–1302. [Google Scholar] [CrossRef]
- Chen, H. Optimization of active sites via crystal phase, composition, and morphology for efficient low-iridium oxygen evolution catalysts. Angew. Chem. 2020. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z. Robust Ti- and Zr-Based Metal-Organic Frameworks for Photocatalysis. Chin. J. Chem. 2017, 35, 135–147. [Google Scholar] [CrossRef]
- Ruano, D.; Díaz-García, M.; Alfayate, A.; Sánchez-Sánchez, M. Nanocrystalline M-MOF-74 as Heterogeneous Catalysts in the Oxidation of Cyclohexene: Correlation of the Activity and Redox Potential. ChemCatChem 2015, 7, 674–681. [Google Scholar] [CrossRef]
- Zheng, Y. Metal-organic frameworks/graphene-based materials: Preparations and applications. Adv. Funct. Mater. 2018, 28, 1804950. [Google Scholar] [CrossRef]
- Kovacic, M. Advanced Oxidation Processes, in Encyclopedia of Water; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 1–15. [Google Scholar]
- Copeland, A.; Lytle, D.A. Measuring the oxidation-reduction potential of important oxidants in drinking water. J. Am. Water Work. Assoc. 2014, 106, E10–E20. [Google Scholar] [CrossRef]
- Navarro, P.; Pellicer, J.A.; Gómez-López, V.M. Degradation of azo dye by an UV/H2O2 advanced oxidation process using an amalgam lamp. Water Environ. J. 2018, 33, 476–483. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Venkatesan, A.; Agarwal, S.R.; Ahamed, N.N.S.A.; Ramakrishna, S. Fabrication of NiO/zirconium oxide nanofibers by electrospinning. Mater. Sci. Eng. C 2014, 45, 369–373. [Google Scholar] [CrossRef]
- Ercan, Ö.; Deniz, S.; Yetimoğlu, E.K.; Aydın, A. Degradation of reactive dyes using advanced oxidation method. CLEAN Soil Air Water 2015, 43, 1031–1036. [Google Scholar] [CrossRef]
- Li, J.; Miao, J.; Duan, X.; Dai, J.; Liu, Q.; Wang, S.; Zhou, W.; Shao, Z. Fine-tuning surface properties of perovskites via nanocompositing with inert oxide toward developing superior catalysts for advanced oxidation. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Sun, W. Ozone catalytic oxidation capacity of Ti-Co@Al2O3 for the treatment of biochemical tailwater from the coal chemical industry. Water Environ. Res. 2020. [Google Scholar] [CrossRef]
- Ficken, K.L.G.; Byrne, P.G. Heavy metal pollution negatively correlates with anuran species richness and distribution in south-eastern Australia. Austral Ecol. 2012, 38, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, Y.; Li, W.; Li, X.; Tian, H.; Wei, X.; Ren, Z.; Han, G. Ultrathin anatase TiO2 nanosheets for high-performance photocatalytic hydrogen production. Small 2017, 13, 1604115. [Google Scholar] [CrossRef]
- Industrial Water Resource Management: Challenges and Opportunities for Corporate Water Stewardship; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018.
- Kakavandi, B.; Roshanak, R.K.; Ahmad, J.J.; Simin, N.; Ahmad, A.; Esrafili, A.; Azari, A. Pb(II) adsorption onto a magnetic composite of activated carbon and superparamagnetic Fe3O4 nanoparticles: Experimental and modeling study. CLEAN Soil Air Water 2015, 43, 1157–1166. [Google Scholar] [CrossRef]
- Mahsa, E.A.; Peyman, N.M.; Mohammad, M.B. Synthesis of a new nanocomposite based-on graphene-oxide for selective removal of Pb2+ ions from aqueous solutions. Polym. Compos. 2018, 40, 730–737. [Google Scholar]
- He, X.; Yang, D.-P.; Zhang, X.; Liu, M.; Kang, Z.; Lin, C.; Jia, N.; Luque, R. Waste eggshell membrane-templated CuO-ZnO nanocomposites with enhanced adsorption, catalysis and antibacterial properties for water purification. Chem. Eng. J. 2019, 369, 621–633. [Google Scholar] [CrossRef]
- SoleimaniLashkenari, M.; Rezaei, S.; Lashkenari, A.S.; Peyravi, M. Synthesis of a Ni-Pt electrocatalyst supported on PRh/ZnO nanocomposites and its electrocatalytic behaviour towards methanol electrooxidation. ChemistrySelect 2019, 4, 13892–13898. [Google Scholar] [CrossRef]
- Tang, X.; Chu, W.; Qian, J.; Lin, J.; Cao, G. Low Temperature synthesis of large-size anatase TiO2 Nanosheets with enhanced photocatalytic activities. Small 2017, 13, 1701964. [Google Scholar] [CrossRef]
- Shumba, M.; Nyokong, T. Characterization and electrocatalytic activity of nanocomposites consisting of nanosized cobalt tetraaminophenoxy phthalocyanine, multi-walled carbon nanotubes and gold nanoparticles. Electroanalysis 2016, 28, 1478–1488. [Google Scholar] [CrossRef]
- Husaon-Edwards, K.A. Water pollution. In The Encyclopedia of Archaeological Sciences; Sandra, L., Varela, L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 4, pp. 1–3. [Google Scholar]
- Wang, M.; Ye, M. James Iocozzia and Zhiqun Lin, Photocatalytic Hydrogen Generation Enabled by Nanostructured TiO2 Materials; Crivener Publishing LLC.: Beverly, MA, USA, 2017. [Google Scholar]
- Chen, J. Ni(OH)2 nanosheet electrocatalyst toward alkaline urea electrolysis for energy-saving acidic hydrogen production. ChemElectroChem 2019, 6, 5313–5320. [Google Scholar] [CrossRef]
- Yan, B.; Chen, Z.; Xu, Y. Amorphous and crystalline 2D polymeric carbon nitride nanosheets for photocatalytic hydrogen/oxygen evolution and hydrogen peroxide production. Chem. Asian J. 2020, 15, 2329–2340. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, H.; Xiong, X.; Wu, M.-F.; Xia, M.; Xie, M.; Zou, J.-P.; Luo, S. Highly efficient charge transfer in CdS-covalent organic framework nanocomposites for stable photocatalytic hydrogen evolution under visible light. Sci. Bull. 2020, 65, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y. Component-tunable rutile-anatase TiO2/reduced graphene oxide nanocomposites for enhancement of electrocatalytic oxygen evolution. ChemNanoMat 2018, 4, 1133–1139. [Google Scholar] [CrossRef]
- Azhar, M.; Arafatb, Y.; Khiadania, M.; Wangc, S.; Shao, Z. Water-stable MOFs-based core-shell nanostructures for advanced oxidation towards environmental remediation. Compos. Part B Eng. 2020, 192, 107985. [Google Scholar] [CrossRef]
- Bharad, P.A.; Nikam, A.V.; Thomas, F.; Gopinath, C.S. CuOx-TiO2 Composites: Electronically Integrated Nanocomposites for Solar Hydrogen Generation. ChemistrySelect 2018, 3, 12022–12030. [Google Scholar] [CrossRef]
- Zsolt, K. Novel Applications and Future Perspectives of Nanocomposites. Nanocomposites for Visible Light-Induced Photocatalysis; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2017; pp. 333–398. [Google Scholar]
- Pap, Z. TiO2/WO3/Au/MWCNT composite materials for photocatalytic hydrogen production: Advantages and draw-backs. Phys. Status Solidi 2012, 249, 2592–2595. [Google Scholar] [CrossRef]
- Tian, G.; Wang, A.; Mu, B.; Kang, Y.; Wang, A. Ag(I)-triggered one-pot synthesis of Ag nanoparticles onto natural nanorods as a multifunctional nanocomposite for efficient catalysis and adsorption. J. Colloid Interface Sci. 2016, 473, 84–92. [Google Scholar] [CrossRef]
- Umapathi, S.; Masud, J.; Swesi, A.T.; Nath, M. FeNi2Se4-Reduced graphene oxide nanocomposite: Enhancing bifunctional electrocatalytic activity for oxygen evolution and reduction through synergistic effects. Adv. Sustain. Syst. 2017, 1, 1700086. [Google Scholar] [CrossRef]
- Siliveri, S.; Chirra, S.; Tyagi, C.; Gandamalla, A.; Adepu, A.K.; Goskula, S.; Gujjula, S.R.; Venkatathri, N. New porous high surface area, TiO2 anatase/SAPO-35 mild brønsted acidic nanocomposite: Synthesis, characterization and studies on it’s enhanced photocatalytic activity. ChemistrySelect 2019, 4, 9135–9142. [Google Scholar] [CrossRef]
- Zhou, S.; Song, C.; Kong, W.; Wang, B.; Kong, Y. Effects of synergetic effect between Co and γ-Fe2O3 in confined silica matrix of MCM-41 on the formation of free radicals for the advanced oxidation technology. Appl. Surf. Sci. 2020, 527, 146853. [Google Scholar] [CrossRef]
- Jiang, H.; Tian, L.; Chen, P.; Bai, Y.; Li, X.; Shu, H.; Luo, X. Efficient antimony removal by self-assembled core-shell nanocomposite of Co3O4@rGO and the analysis of its adsorption mechanism. Environ. Res. 2020, 187, 109657. [Google Scholar] [CrossRef]
- Neatu, S. Degenerated TiO2 semiconductor modified with Ni and Zn as efficient photocatalysts for photocatalytic water splitting reaction. ChemCatChem 2020. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chen, H.; Wang, R.; Shang, Y.; Zhang, Q.; Li, W.; Zhang, G.; Su, J.; Dinh, C.-T.; De Arquer, F.P.G.; et al. 0D-2D quantum dot: Metal dichalcogenide nanocomposite photocatalyst achieves efficient hydrogen generation. Adv. Mater. 2017, 29, 1605646. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Wang, X.-L.; Li, S.; Huang, W.; Dong, L.; Liu, C.; Li, Y.-F.; Lan, Y.-Q. Molybdenum disulfide/nitrogen-doped reduced graphene oxide nanocomposite with enlarged interlayer spacing for electrocatalytic hydrogen evolution. Adv. Energy Mater. 2016, 6, 1600116. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Troppová, I.; Prostějovský, T.; Žebrák, R. Degradation of styrene from waste gas stream by advanced oxidation processes. CLEAN Soil Air Water 2019, 47. [Google Scholar] [CrossRef]
- Liu, C. High energy and power lithium-ion capacitors based on Mn3O4/3D-graphene as anode and activated polyaniline-derived carbon nanorods as cathode. Chem. Eng. J. 2019, 370, 1485–1492. [Google Scholar] [CrossRef]
- Sharma, G. Polyacrylamide@Zr(IV) vanadophosphate nanocomposite: Ion exchange properties, antibacterial activity, and photocatalytic behavior. J. Ind. Eng. Chem. 2016, 33, 201–208. [Google Scholar] [CrossRef]









| Oxidant | Oxidation Potential (V) (Hydrogen Standard) |
|---|---|
| F2 | 3.03 |
| •OH | 2.80 |
| SO4−• | 2.60 |
| •O | 2.42 |
| O3 | 2.07 |
| S2O82− | 2.01 |
| H2O2 | 1.77 |
| Cl2 | 1.36 |
| O2 | 0.40 |
| Sample Details | Average Fiber Diameters before Calcination (nm) | Average Fiber Diameters after Calcination (nm) |
|---|---|---|
| PVA (11 wt.%) | 242 ± 35 | Not applicable |
| PVA/NiO-ZrO2(90/10) | 213 ± 44 | 30 ± 6 |
| PVA/NiO-ZrO2(80/20) | 195 ± 74 | 25 ± 9 |
| PVA/NiO-ZrO2(50/50) | 145 ± 30 | 106 ± 25 |
| Isotherm Model | Temperature (°C) | ||
|---|---|---|---|
| 20 | 35 | 50 | |
| Langmuir Isotherm | |||
| qm (mg/g) | 58.82 | 62.5 | 71.42 |
| KL (L/mg) | 0.068 | 0.07 | 0.06 |
| R2 | 0.991 | 0.989 | 0.982 |
| RL | 0.04–0.22 | 0.045–0.23 | 0.052–0.025 |
| Freundlich Isotherm | |||
| KF (mg/g (L/mg) | 16 | 17.88 | 22.95 |
| n | 4.11 | 4.34 | 5.34 |
| RL | 0.959 | 0.958 | 0.931 |
| Temkin Isotherm | |||
| KT | 2.6 | 3.73 | 16 |
| B1 | 8.9 | 8.8 | 7.44 |
| RL | 0.928 | 0.916 | 0.864 |
| Dubinin–Radushkevich | |||
| qm (mol/g) | 5 × 10−4 | 4.8 × 10−4 | 4.5 × 10−4 |
| D (mol2/KJ2) | 0.0025 | 0.0022 | 0.0021 |
| E (KJ/mol) | 14.1 | 15 | 15.5 |
| RL | 0.953 | 0.944 | 0.898 |
| Types | Nanomaterials | Performance | Conditions | References |
|---|---|---|---|---|
| Advanced Oxidation | TiO2 | Degradation of methylene blue (MB) | 25 min of sunlight | [85] |
| TiO2 | Degradation of methylene blue (MB) | pH = 7, UVC (95W, λ = 254 nm) | [17] | |
| TiO2 | Catalytic reduction of Cr5+ | Under 200–800 nm light irradiation | [71] | |
| Ti-Co@γAl2O3 | Removal of total phenol and total organic carbon | pH = 8.2, ozone ventilation is 30 mg/min | [63] | |
| γ-Fe2O3@Co-MCM-41 | Degradation of Orange II | Activation PMS | [86] | |
| MOFs-NiP | Degradation of Rhodamine B (RhB) | Activation PMS | [79] | |
| NiCo2O4 | Degradation of Rhodamine B (RhB) | Neutral pH, current density 10 Ma·cm−2, activated PMS | [40] | |
| La1.15FeO3 | Oxidative degradation of methyl orange | pH = 2.8–3 | [62] | |
| Catalytic Adsorption | Fe2O3@C | Adsorption of Pb2+ in water | pH = 6, Temperature is 20 °C, time is 60 min | [67] |
| CuO-ZnO | Adsorption and catalytic reduction of 4-nitrophenol (4-NP) and strong antibacterial activity | (UV)λ = 498 nm | [69] | |
| GO | Adsorption of Pb2+, Cr2+, Cd2+ in water | pH = 5–6 | [68] | |
| MOFs@GO (2:1) | Adsorption of methylene blue (MB) and uranium (U) | With the increase of temperature, the adsorption performance is enhanced | [56] | |
| Co3O4@rGO | Adsorption of Sb(III) and Sb(V) | pH = 9 | [87] | |
| GO-Ag | Adsorption and removal of 2-nitroaniline (2-NA) and vat dyes (MG, MO, and EV) | pH = 9 | [48] | |
| PVA/GO-SA | Adsorption of Pb2+ in water | When the GO content is 5 wt% | [17] | |
| PAL/PANI/AgNPs | Adsorption and catalytic reduction of Congo Red (CR), Adsorption of H2PO4 | pH = 7.4 | [83] | |
| Nanomaterials | Function | References | ||
| TiO2 Nanosheets | Photocatalytic hydrogen production | [17,74] | ||
| CuOx-TiO2 | Solar hydrolysis | [80] | ||
| Ni-Zn/TiO2 (9:1) | Photocatalytic hydrogen production | [88] | ||
| TiO2/WO3/Au/MWCNT | Photocatalytic hydrogen production | [82] | ||
| CdS-CTF-1 | Photocatalytic hydrogen production | [77] | ||
| 0D ZAIS CQD and 2D MoS2 | Photocatalytic hydrogen production | [89] | ||
| MoS2/Graphene | Electrocatalytic oxygen production | [50] | ||
| CoNiOx/rGO | Electrocatalytic oxygen production | [20] | ||
| FeNi2Se4-NrGO | Bifunctional catalyst for OER and ORR | [80] | ||
| MoS2/N-RGO-180 | Electrocatalytic oxygen production | [90] | ||
| Fe(Ni)-MOF | Electrocatalytic oxygen production | [33] | ||
| Manganese copper sulfide (MCS) | Photocatalytic and electrocatalytic hydrolysis | [39] | ||
| PRh/ZnO/Ni/Pt | Methanol oxidation | [68] | ||
| Type of Oxidation Reaction | Advantages | Limitations | Features |
|---|---|---|---|
| Photooxidation |
| The utilization rate of solar energy is low, and only less than 5% of ultraviolet light in sunlight can be used to excite photo-generated electrons and photo-generated holes | It mainly produces strong oxidizing free radicals under the irradiation of visible light (ultraviolet light), which has a good degradation effect on refractory organic wastewater |
| Electrooxidation |
| High-performance electrode materials need not only high hydrogen evolution potential and two-electron oxygen reduction reaction activity but also good stability and corrosion resistance | It regulates the generation of ·OH during the electrode reaction process to achieve the purpose of efficient oxidation. It is widely used in the treatment of organic pollutants and heavy metals |
| Fenton oxidation |
|
| Under acidic conditions (pH = 2~5), it reacts to produce highly oxidizing ·OH, ·OH can degrade and mineralize most organic matter |
| Ozone oxidation |
|
| It can be decomposed at room temperature to produce ·OH and monoatomic oxygen, which is effective in decolorization, deodorization, and deodorization of sewage, and removal of pollutants |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomaterials 2020, 10, 1719. https://doi.org/10.3390/nano10091719
Su R, Xie C, Alhassan SI, Huang S, Chen R, Xiang S, Wang Z, Huang L. Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials. Nanomaterials. 2020; 10(9):1719. https://doi.org/10.3390/nano10091719
Chicago/Turabian StyleSu, Rongkui, Chuyue Xie, Sikpaam Issaka Alhassan, Shunhong Huang, Runhua Chen, Siyuan Xiang, Zhenxing Wang, and Lei Huang. 2020. "Oxygen Reduction Reaction in the Field of Water Environment for Application of Nanomaterials" Nanomaterials 10, no. 9: 1719. https://doi.org/10.3390/nano10091719