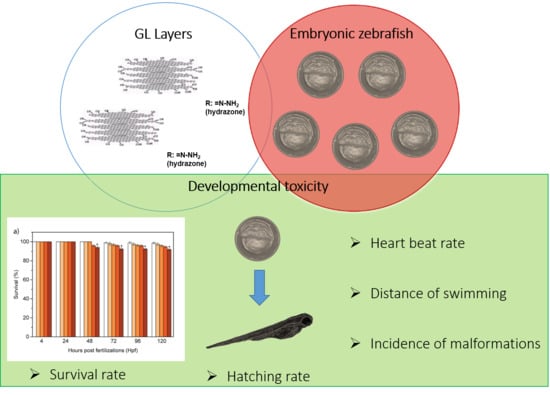
Graphene-Like Layers from Carbon Black: In Vivo Toxicity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Gl Layers
2.2. Material Characterization
Atomic Force Microscopy (AFM)
2.3. Biological Studies
2.3.1. Zebrafish Culture
2.3.2. In Vivo Toxicity
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. GL Layers Chemico-Physical Characteristics
3.2. Biotoxicity Assessment
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bischoff, D.; Eich, M.; Varlet, A.; Simonet, P.; Overweg, H.C.; Ensslin, K.; Ihn, T. Graphene nano-heterostructures for quantum devices. Mater. Today 2016, 19, 375–381. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent advances in sensing applications of graphene assemblies and their composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, Z.; Jia, D. Heteroatom-doped graphene as electrocatalysts for air cathodes. Mater. Horiz. 2017, 4, 7–19. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Liang, H.W.; Feng, X.; Thomas, A. 2d porous carbons prepared from layered organic-inorganic hybrids and their use as oxygen-reduction electrocatalysts. Adv. Mater. 2017, 29, 1700707. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-based microbial-fuel-cell electrodes: From conductive supports to active catalysts. Adv. Mater. 2017, 29, 1602547. [Google Scholar] [CrossRef]
- Perreault, F.; Fonseca de Faria, A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Dasgupta, A.; Rajukumar, L.P.; Rotella, C.; Lei, Y.; Terrones, M. Covalent three-dimensional networks of graphene and carbon nanotubes: Synthesis and environmental applications. Nano Today 2017, 12, 116–135. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [Green Version]
- Xie, C.; Wang, Y.; Zhang, Z.-X.; Wang, D.; Luo, L.-B. Graphene/semiconductor hybrid heterostructures for optoelectronic device applications. Nano Today 2018, 19, 41–83. [Google Scholar] [CrossRef]
- D’Amora, M.; Giordani, S. 7—Carbon nanomaterials for nanomedicine. In Smart Nanoparticles for Biomedicine; Ciofani, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 103–113. [Google Scholar]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of graphene-based nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity effects of graphene and single-wall carbon nanotubes in neural phaeochromocytoma-derived pc12 cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, H.; Sui, J.; Duan, H.; Chen, W.N.; Ching, C.B. Cytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma hepg2 cells: An itraq-coupled 2d lc-ms/ms proteome analysis. Toxicol. Sci. 2012, 126, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chng, E.L.; Chua, C.K.; Pumera, M. Graphene oxide nanoribbons exhibit significantly greater toxicity than graphene oxide nanoplatelets. Nanoscale 2014, 6, 10792–10797. [Google Scholar] [CrossRef] [Green Version]
- Dasmahapatra, A.K.; Dasari, T.P.S.; Tchounwou, P.B. Graphene-based nanomaterials toxicity in fish. Rev. Environ. Contam. Toxicol. 2019, 247, 1–58. [Google Scholar]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Bangeppagari, M.; Park, S.H.; Kundapur, R.R.; Lee, S.J. Graphene oxide induces cardiovascular defects in developing zebrafish (danio rerio) embryo model: In-vivo toxicity assessment. Sci. Total Environ. 2019, 673, 810–820. [Google Scholar] [CrossRef]
- Li, R.; Guiney, L.M.; Chang, C.H.; Mansukhani, N.D.; Ji, Z.; Wang, X.; Liao, Y.P.; Jiang, W. Surface oxidation of graphene oxide determines membrane damage, lipid peroxidation, and cytotoxicity in macrophages in a pulmonary toxicity model. ACS Nano 2018, 12, 1390–1402. [Google Scholar] [CrossRef]
- Firkowska-Boden, I.; Zhang, X.; Jandt, K.D. Biocompatibility: Controlling protein adsorption through nanostructured polymeric surfaces (adv. Healthcare mater. 1/2018). Adv. Healthc. Mater. 2018, 7, 1870001. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, A.; Panchakarla, L.S.; Sadanandan, A.R.; Ashokan, A.; Chandran, P.; Girish, C.M.; Menon, D.; Nair, S.V.; Rao, C.N.; Koyakutty, M. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small 2012, 8, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.; Park, S.H.; Kim, K.; Kundapur, R.R.; Lee, S.J. Pristine graphene induces cardiovascular defects in zebrafish (danio rerio) embryogenesis. Environ. Pollut. 2018, 243, 246–254. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, L.; Pretti, C.; Gabriel, B.; Marques, P.; Freitas, R.; Neto, V. An overview of graphene materials: Properties, applications and toxicity on aquatic environments. Sci. Total Environ. 2018, 631–632, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Hougaard, K.S.; Kishimoto, A.; Honda, K. Reproductive and developmental toxicity of carbon-based nanomaterials: A literature review. Nanotoxicology 2016, 10, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Wang, Z. Toxicity of mixtures of zinc oxide and graphene oxide nanoparticles to aquatic organisms of different trophic level: Particles outperform dissolved ions. Nanotoxicology 2018, 12, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Clemente, Z.; Silva, G.H.; de Souza Nunes, M.C.; Martinez, D.S.T.; Maurer-Morelli, C.V.; Thomaz, A.A.; Castro, V. Exploring the mechanisms of graphene oxide behavioral and morphological changes in zebrafish. Environ. Sci. Pollut. Res. Int. 2019, 26, 30508–30523. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Kurtycz, P.; Olszyna, A.R. Recent advances in graphene family materials toxicity investigations. J. Nanopart. Res. 2012, 14, 1320. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hu, P.; Zhang, L.; Huang, S.; Luo, L.; Huang, C. Toxicity of graphene oxide and multi-walled carbon nanotubes against human cells and zebrafish. Sci. China Chem. 2012, 55, 2209–2216. [Google Scholar] [CrossRef]
- Singh, Z. Applications and toxicity of graphene family nanomaterials and their composites. Nanotechnol. Sci. Appl. 2016, 9, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.G.; Zhou, R.; Jiang, D.; Song, J.E.; Xu, Q.; Si, J.; Chen, Y.P.; Zhou, X.; Gan, L.; Li, J.Z.; et al. Toxicity of graphene quantum dots in zebrafish embryo. Biomed. Environ. Sci. 2015, 28, 341–351. [Google Scholar] [PubMed]
- Pecoraro, R.; D’Angelo, D.; Filice, S.; Scalese, S.; Capparucci, F.; Marino, F.; Iaria, C.; Guerriero, G.; Tibullo, D.; Scalisi, E.M.; et al. Toxicity evaluation of graphene oxide and titania loaded nafion membranes in zebrafish. Front. Physiol. 2018, 8, 1039. [Google Scholar] [CrossRef] [PubMed]
- Gollavelli, G.; Ling, Y.C. Multi-functional graphene as an in vitro and in vivo imaging probe. Biomaterials 2012, 33, 2532–2545. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Cho, H.-J.; Choi, M.; Lee, W.S.; Chung, B.; Lee, J.-S. In vivo toxicity assessment of angiogenesis and the live distribution of nano-graphene oxide and its pegylated derivatives using the developing zebrafish embryo. Carbon 2015, 93, 431–440. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q. Molecular mechanisms of developmental toxicity induced by graphene oxide at predicted environmental concentrations. Environ. Sci. Technol. 2017, 51, 7861–7871. [Google Scholar] [CrossRef]
- Clemente, Z.; Castro, V.; Franqui, L.S.; Silva, C.A.; Martinez, D.S.T. Nanotoxicity of graphene oxide: Assessing the influence of oxidation debris in the presence of humic acid. Environ. Pollut. 2017, 225, 118–128. [Google Scholar] [CrossRef]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q.; Sun, H.; Wang, C.J.; Li, X.F. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific nanotoxicity of graphene oxide during zebrafish embryogenesis. Nanotoxicology 2016, 10, 42–52. [Google Scholar] [CrossRef]
- D’Amora, M.; Camisasca, A.; Lettieri, S.; Giordani, S. Toxicity assessment of carbon nanomaterials in zebrafish during development. Nanomaterials 2017, 7, 414. [Google Scholar] [CrossRef] [Green Version]
- D’Amora, M.; Lamberti, A.; Fontana, M.; Giordani, S. Toxicity assessment of laser-induced graphene by zebrafish during development. J. Phys. Mater. 2020, 3, 034008. [Google Scholar] [CrossRef]
- Mao, L.; Hu, M.; Pan, B.; Xie, Y.; Petersen, E.J. Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation. Part. Fibre Toxicol. 2016, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.Y.; Xia, T.; Liu, S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef] [PubMed]
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfe, M.; Gargiulo, V.; Di Capua, R.; Chiarella, F.; Rouzaud, J.N.; Vergara, A.; Ciajolo, A. Wet chemical method for making graphene-like films from carbon black. ACS Appl. Mater. Interfaces 2012, 4, 4491–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargiulo, V.; Alfano, B.; Capua, R.D.; Alfé, M.; Vorokhta, M.; Polichetti, T.; Massera, E.; Miglietta, M.L.; Schiattarella, C.; Francia, G.D. Graphene-like layers as promising chemiresistive sensing material for detection of alcohols at low concentration. J. Appl. Phys. 2018, 123, 024503. [Google Scholar] [CrossRef]
- Di Capua, R.; Gargiulo, V.; Alfè, M.; De Luca, G.M.; Skála, T.; Mali, G.; Pezzella, A. Eumelanin graphene-like integration: The impact on physical properties and electrical conductivity. Front. Chem. 2019, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, V.; Alfè, M.; Capua, R.D.; Togna, A.R.; Cammisotto, V.; Fiorito, S.; Musto, A.; Navarra, A.; Parisi, S.; Pezzella, A. Supplementing π-systems: Eumelanin and graphene-like integration towards highly conductive materials for the mammalian cell culture bio-interface. J. Mater. Chem. B 2015, 3, 5070–5079. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Di Capua, R. Tuning the surface morphology of self-assembled graphene-like thin films through ph variation. Appl. Surf. Sci. 2015, 353, 628–635. [Google Scholar] [CrossRef]
- Mrózek, O.; Melounková, L.; Smržová, D.; Machálková, A.; Vinklárek, J.; Němečková, Z.; Komárková, B.; Ecorchard, P. Salt-washed graphene oxide and its cytotoxicity. J. Hazard. Mater. 2020, 398, 123114. [Google Scholar] [CrossRef]
- Zhen, X.; Ng, W.C.; Tong, Y.W.; Dai, Y.; Neoh, K.G.; Wang, C.H. Toxicity assessment of carbon black waste: A by-product from oil refineries. J. Hazard. Mater. 2017, 321, 600–610. [Google Scholar] [CrossRef]
- Olivi, M.; Alfe, M.; Gargiulo, V.; Valle, F.; Mura, F.; Di Giosia, M.; Rapino, S.; Palleschi, C.; Uccelletti, D.; Fiorito, S. Antimicrobial properties of graphene-like nanoparticles: Coating effect on staphylococcus aureus. J. Nanopart. Res. 2016, 18, 358. [Google Scholar] [CrossRef]
- D’Amora, M.; Giordani, S. The utility of zebrafish as a model for screening developmental neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amora, M.; Camisasca, A.; Boarino, A.; Arpicco, S.; Giordani, S. Supramolecular functionalization of carbon nano-onions with hyaluronic acid-phospholipid conjugates for selective targeting of cancer cells. Colloids Surf. B Biointerfaces 2020, 188, 110779. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, V.; Alfè, M.; Lisi, L.; Manfredi, C.; Volino, S.; Di Natale, F. Colloidal carbon-based nanoparticles as heavy metal adsorbent in aqueous solution: Cadmium removal as a case study. Water Air Soil Pollut. 2017, 228, 192. [Google Scholar] [CrossRef] [Green Version]
- D’Amora, M.; Cassano, D.; Pocoví-Martínez, S.; Giordani, S.; Voliani, V. Biodistribution and biocompatibility of passion fruit-like nano-architectures in zebrafish. Nanotoxicology 2018, 12, 914–922. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
d’Amora, M.; Alfe, M.; Gargiulo, V.; Giordani, S. Graphene-Like Layers from Carbon Black: In Vivo Toxicity Assessment. Nanomaterials 2020, 10, 1472. https://doi.org/10.3390/nano10081472
d’Amora M, Alfe M, Gargiulo V, Giordani S. Graphene-Like Layers from Carbon Black: In Vivo Toxicity Assessment. Nanomaterials. 2020; 10(8):1472. https://doi.org/10.3390/nano10081472
Chicago/Turabian Styled’Amora, Marta, Michela Alfe, Valentina Gargiulo, and Silvia Giordani. 2020. "Graphene-Like Layers from Carbon Black: In Vivo Toxicity Assessment" Nanomaterials 10, no. 8: 1472. https://doi.org/10.3390/nano10081472