Hydrothermal Treatment of Wheat Bran under Mild Acidic or Alkaline Conditions for Enhanced Polyphenol Recovery and Antioxidant Activity
Abstract
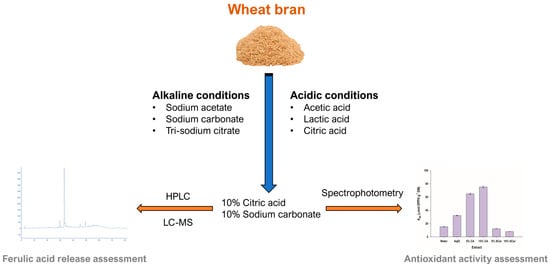
:1. Introduction
2. Results and Discussion
2.1. Effect of Mild Acid Catalysis
2.2. Effect of Mild Alkaline Catalysis
2.3. Comparative Extraction Efficiency Appraisal
2.4. Severity Effects
2.5. Polyphenolic Composition—Tentative Polyphenol Release Mechanism
2.6. Antioxidant Characteristics
3. Materials and Methods
3.1. Chemicals
3.2. Wheat Bran
3.3. Reference Alkaline Hydrolysis
3.4. Hydrothermal Treatments
3.5. Process Severity Assessment
3.6. Determination of Total Polyphenols and Antioxidant Activity
3.7. Liquid Chromatography–Diode Array–Mass Spectrometry (LC–DAD–MS)
3.8. Statistical Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.; Lourenço-Lopes, C.; Prieto, M.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef] [PubMed]
- Ouro-Salim, O.; Guarnieri, P. Circular economy of waste in agrifood supply chain: A review. Thunderbird 2022, 64, 333–348. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass 2023, 3, 123–137. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The potential of selected agri-food loss and waste to contribute to a circular economy: Applications in the food, cosmetic and pharmaceutical industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Varatharajan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Martínez-Montaño, E.; Arrizon, J.; Guerrero, A.; Benitez-Hernández, A. Antioxidant molecules from plant waste: Extraction techniques and biological properties. Processes 2020, 8, 1566. [Google Scholar] [CrossRef]
- Galanakis, C.M. Sustainable applications for the valorization of cereal processing by-products. Foods 2022, 11, 241. [Google Scholar] [CrossRef]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the valorisation and functionalization of by-products and wastes from cereal-based processing industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef]
- Katileviciute, A.; Plakys, G.; Budreviciute, A.; Onder, K.; Damiati, S.; Kodzius, R. A sight to wheat bran: High value-added products. Biomolecules 2019, 9, 887. [Google Scholar] [CrossRef]
- Prueckler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Hoeltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT-Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- de Oliveira Silva, E.; Batista, R. Ferulic acid and naturally occurring compounds bearing a feruloyl moiety: A review on their structures, occurrence, and potential health benefits. Comp. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-x.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, S.; Rameshpathy, M. Valorization of agro-waste residues into bio-vanillin a comprehensive review. Ind. Crops Prod. 2023, 205, 117522. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H. The role of bioconversion processes to enhance bioaccessibility of polyphenols in rice. Food Biosci. 2020, 35, 100605. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Pazo-Cepeda, M.V.; Aspromonte, S.G.; Alonso, E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021, 44, 101374. [Google Scholar] [CrossRef]
- Rico, D.; Villaverde, A.; Martinez-Villaluenga, C.; Gutierrez, A.L.; Caballero, P.a.; Ronda, F.; Peñas, E.; Frias, J.; Martin Diana, A.B. Application of autoclave treatment for development of a natural wheat bran antioxidant ingredient. Foods 2020, 9, 781. [Google Scholar] [CrossRef]
- Cherif, M.M.; Grigorakis, S.; Halahlah, A.; Loupassaki, S.; Makris, D.P. High-efficiency extraction of phenolics from wheat waste biomass (bran) by combining deep eutectic solvent, ultrasound-assisted pretreatment and thermal treatment. Environ. Process. 2020, 7, 845–859. [Google Scholar] [CrossRef]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Kinetic modelling for flavanol extraction from red grape (Vitis vinifera L.) pomace using aqueous organic acid solutions. Int. Food Res. J. 2014, 21, 1919. [Google Scholar]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. A comparative evaluation of aqueous natural organic acid media for the efficient recovery of flavonoids from red grape (Vitis vinifera) pomace. Waste Biomass Valoriz. 2015, 6, 391–400. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Ma, R.; Xu, X.; Zhao, C.; Wang, Z.; Chen, F.; Hu, X. Effect of energy density and citric acid concentration on anthocyanins yield and solution temperature of grape peel in microwave-assisted extraction process. J. Food Eng. 2012, 109, 274–280. [Google Scholar] [CrossRef]
- Benfica, J.; Morais, E.S.; Miranda, J.S.; Freire, M.G.; de Sousa, R.d.C.S.; Coutinho, J.A. Aqueous solutions of organic acids as effective solvents for levodopa extraction from Mucuna pruriens seeds. Sep. Purif. Technol. 2021, 274, 119084. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Pappas, V.M.; Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Pressurized liquid extraction of polyphenols and anthocyanins from saffron processing waste with aqueous organic acid solutions: Comparison with stirred-tank and ultrasound-assisted techniques. Sustainability 2021, 13, 12578. [Google Scholar] [CrossRef]
- Arranz, S.; Calixto, F.S. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol release from wheat bran using ethanol-based organosolv treatment and acid/alkaline catalysis: Process modeling based on severity and response surface optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yu, L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT-Food Sci. Technol. 2004, 37, 717–721. [Google Scholar] [CrossRef]
- Abozed, S.S.; El-Kalyoubi, M.; Abdelrashid, A.; Salama, M.F. Total phenolic contents and antioxidant activities of various solvent extracts from whole wheat and bran. Annals Agric. Sci. 2014, 59, 63–67. [Google Scholar] [CrossRef]
- Kottaras, P.; Koulianos, M.; Makris, D.P. Low-Transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Liu, C.; Zheng, X.; Liu, B. Enhancing antioxidant activity and antiproliferation of wheat bran through steam flash explosion. J. Food Sci. Technol. 2016, 53, 3028–3034. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotech. 2010, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Pazo-Cepeda, V.; Benito-Román, Ó.; Navarrete, A.; Alonso, E. Valorization of wheat bran: Ferulic acid recovery using pressurized aqueous ethanol solutions. Waste Biomass Valoriz. 2020, 11, 4701–4710. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Finger-Teixeira, A.; Rodrigues Mota, T.; Salvador, V.H.; Moreira-Vilar, F.C.; Correa Molinari, H.B.; Craig Mitchell, R.A.; Marchiosi, R.; Ferrarese-Filho, O.; Dantas dos Santos, W. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotech. J. 2015, 13, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E. Ferulic acid: An antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotech. 2004, 24, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Buranov, A.U.; Mazza, G. Lignin in straw of herbaceous crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Linh, T.N.; Fujita, H.; Sakoda, A. Release kinetics of esterified p-coumaric acid and ferulic acid from rice straw in mild alkaline solution. Bioresour. Technol. 2017, 232, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar] [CrossRef]
- López-Perea, P.; Guzmán-Ortiz, F.; Román-Gutiérrez, A.; Castro-Rosas, J.; Gómez-Aldapa, C.; Rodríguez-Marín, M.; Falfán-Cortés, R.; González-Olivares, L.; Torruco-Uco, J. Bioactive compounds and antioxidant activity of wheat bran and barley husk in the extracts with different polarity. Int. J. Food Prop. 2019, 22, 646–658. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Kylli, P.; Nousiainen, P.; Biely, P.; Sipilä, J.; Tenkanen, M.; Heinonen, M. Antioxidant potential of hydroxycinnamic acid glycoside esters. J. Agric. Food Chem. 2008, 56, 4797–4805. [Google Scholar] [CrossRef]
- Ohta, T.; Nakano, T.; Egashira, Y.; Sanada, H. Antioxidant activity of ferulic acid β-glucuronide in the LDL oxidation system. Biosci. Biotech. Biochem. 1997, 61, 1942–1943. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philosoph. Trans. Royal Soc. London. Series A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Kaltsa, O.; Bozinou, E.; Lalas, S.; Makris, D.P. Saffron processing wastes as a bioresource of high-value added compounds: Development of a green extraction process for polyphenol recovery using a natural deep eutectic solvent. Antioxidants 2019, 8, 586. [Google Scholar] [CrossRef] [PubMed]





| Extraction Medium | C (% w/v) | Cmol (mol L−1) | pH |
|---|---|---|---|
| Acetic acid | 0.5 | 0.083 | 2.46 |
| 1.0 | 0.167 | 2.40 | |
| 2.5 | 0.416 | 2.37 | |
| 5.0 | 0.833 | 2.33 | |
| Lactic acid | 0.5 | 0.056 | 2.70 |
| 1.0 | 0.111 | 2.67 | |
| 2.5 | 0.278 | 2.40 | |
| 5.0 | 0.555 | 2.10 | |
| Citric acid | 0.5 | 0.026 | 2.73 |
| 1.0 | 0.052 | 2.52 | |
| 2.5 | 0.130 | 2.36 | |
| 5.0 | 0.260 | 2.19 | |
| Sodium carbonate | 0.5 | 0.047 | 11.25 |
| 1.0 | 0.094 | 11.34 | |
| 2.5 | 0.236 | 11.39 | |
| 5.0 | 0.472 | 11.49 | |
| Sodium acetate | 0.5 | 0.061 | 7.35 |
| 1.0 | 0.122 | 7.64 | |
| 2.5 | 0.305 | 7.88 | |
| 5.0 | 0.610 | 8.90 | |
| Trisodium citrate | 0.5 | 0.019 | 7.98 |
| 1.0 | 0.039 | 8.12 | |
| 2.5 | 0.097 | 8.58 | |
| 5.0 | 0.194 | 8.62 |
| Solvent (% w/v) | YTP (mg FAE g−1 DM) | |||||
|---|---|---|---|---|---|---|
| 3 h | sd | 6 h | sd | 24 h | sd | |
| Water | 3.33 a | 0.09 | 3.61 a | 0.05 | 3.25 a | 0.03 |
| Acetic acid | ||||||
| 0.5 | 2.78 b | 0.04 | 2.64 b | 0.08 | 2.94 b | 0.04 |
| 1.0 | 2.67 b | 0.07 | 2.85 b | 0.07 | 3.26 a | 0.05 |
| 2.5 | 3.31 a | 0.06 | 3.36 c | 0.05 | 3.57 c | 0.06 |
| 5.0 | 3.78 c | 0.10 | 3.93 d | 0.10 | 4.13 d | 0.03 |
| Lactic acid | ||||||
| 0.5 | 2.73 b | 0.04 | 2.91 b | 0.08 | 3.58 c | 0.07 |
| 1.0 | 3.00 d | 0.06 | 3.43 c | 0.05 | 4.19 d | 0.03 |
| 2.5 | 3.05 d | 0.05 | 3.98 d | 0.04 | 4.65 e | 0.04 |
| 5.0 | 4.22 e | 0.12 | 4.58 e | 0.11 | 5.82 f | 0.08 |
| Citric acid | ||||||
| 0.5 | 2.81 b | 0.11 | 4.01 d | 0.11 | 5.49 g | 0.05 |
| 1.0 | 3.33 a | 0.06 | 3.52 a | 0.07 | 5.49 g | 0.10 |
| 2.5 | 3.76 c | 0.05 | 4.48 e | 0.09 | 9.27 h | 0.12 |
| 5.0 | 5.09 f | 0.11 | 6.07 f | 0.11 | 18.77 i | 0.14 |
| Solvent (% w/v) | YTP (mg FAE g−1 DM) | |||||
|---|---|---|---|---|---|---|
| 3 h | sd | 6 h | sd | 24 h | sd | |
| Water | 3.33 a | 0.07 | 3.61 a | 0.01 | 3.25 a | 0.03 |
| Sodium carbonate | ||||||
| 0.5 | 10.48 b | 0.05 | 13.01 b | 0.26 | 12.61 b | 0.02 |
| 1.0 | 14.98 c | 0.22 | 13.39 b | 0.20 | 14.41 c | 0.20 |
| 2.5 | 13.64 d | 0.12 | 17.79 c | 0.19 | 20.84 d | 0.40 |
| 5.0 | 15.72 e | 0.14 | 19.38 d | 0.21 | 22.33 e | 0.31 |
| Sodium acetate | ||||||
| 0.5 | 2.44 f | 0.06 | 1.76 e | 0.05 | 2.84 f | 0.11 |
| 1.0 | 1.54 g | 0.06 | 2.42 f | 0.07 | 4.17 g | 0.14 |
| 2.5 | 2.51 f | 0.10 | 2.46 f | 0.01 | 3.90 a | 0.10 |
| 5.0 | 2.77 h | 0.06 | 3.06 g | 0.09 | 4.19 g | 0.03 |
| Sodium citrate tribasic | ||||||
| 0.5 | 3.22 a | 0.08 | 3.47 a | 0.14 | 5.39 h | 0.02 |
| 1.0 | 5.86 i | 0.01 | 6.58 h | 0.12 | 3.33 a | 0.04 |
| 2.5 | 2.49 f | 0.04 | 2.63 i | 0.01 | 3.36 a | 0.11 |
| 5.0 | 1.61 g | 0.07 | 1.16 j | 0.09 | 2.69 f | 0.09 |
| C (% w/v) | t (min) | CSF’ | YTP (mg FAE g−1 DM) | ||||
|---|---|---|---|---|---|---|---|
| CA | SCar | CA | sd | SCar | sd | ||
| 2.5 | 180 | 6.60 | 6.30 | 3.76 a | 0.05 | 13.64 a | 0.12 |
| 300 | 6.90 | 6.60 | 4.48 b | 0.09 | 17.79 b | 0.19 | |
| 1440 | 7.50 | 7.20 | 9.27 c | 0.12 | 20.84 c | 0.40 | |
| 5.0 | 180 | 6.77 | 6.31 | 5.09 d | 0.11 | 15.72 d | 0.14 |
| 300 | 7.07 | 6.61 | 6.07 e | 0.11 | 19.38 c | 0.21 | |
| 1440 | 7.67 | 7.21 | 18.77 f | 0.14 | 22.33 e | 0.31 | |
| 10.0 | 180 | 7.47 | 6.64 | 6.16 e | 0.09 | 17.24 b | 0.11 |
| 300 | 7.77 | 6.94 | 8.93 c | 0.17 | 19.65 c | 0.14 | |
| 1440 | 8.37 | 7.54 | 23.76 g | 0.32 | 23.60 e | 0.19 | |
| Extraction Medium | Extraction Yield (μg g−1 DM) * | ||
|---|---|---|---|
| Ferulic Acid | Ferulate Derivative | Total | |
| Alkaline hydrolysis | 2158.61 ± 112.02 a | 210.44 ± 5.43 a | 2369.05 a |
| Water | 37.22 ± 2.65 b | 18.83 ± 0.50 b | 56.06 b |
| 60% (v/v) Ehanol | 32.62 ± 2.52 b | 19.16 ± 1.56 b | 55.79 b |
| 10% (w/v) Citric acid | 344.52 ± 3.55 c | 1930.70 ± 58.46 c | 2275.22 c |
| 10% (w/v) Sodium carbonate | 1822.97 ± 16.66 d | 232.04 ± 2.78 d | 2055.01 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadaki, E.; Grigorakis, S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P. Hydrothermal Treatment of Wheat Bran under Mild Acidic or Alkaline Conditions for Enhanced Polyphenol Recovery and Antioxidant Activity. Molecules 2024, 29, 1193. https://doi.org/10.3390/molecules29061193
Papadaki E, Grigorakis S, Palaiogiannis D, Lalas SI, Mitlianga P. Hydrothermal Treatment of Wheat Bran under Mild Acidic or Alkaline Conditions for Enhanced Polyphenol Recovery and Antioxidant Activity. Molecules. 2024; 29(6):1193. https://doi.org/10.3390/molecules29061193
Chicago/Turabian StylePapadaki, Eirini, Spyros Grigorakis, Dimitrios Palaiogiannis, Stavros I. Lalas, and Paraskevi Mitlianga. 2024. "Hydrothermal Treatment of Wheat Bran under Mild Acidic or Alkaline Conditions for Enhanced Polyphenol Recovery and Antioxidant Activity" Molecules 29, no. 6: 1193. https://doi.org/10.3390/molecules29061193