Atractylone in the Atractylodes macrocephala Rhizoma Essential Oil and Its Anti-Inflammatory Activity
Abstract
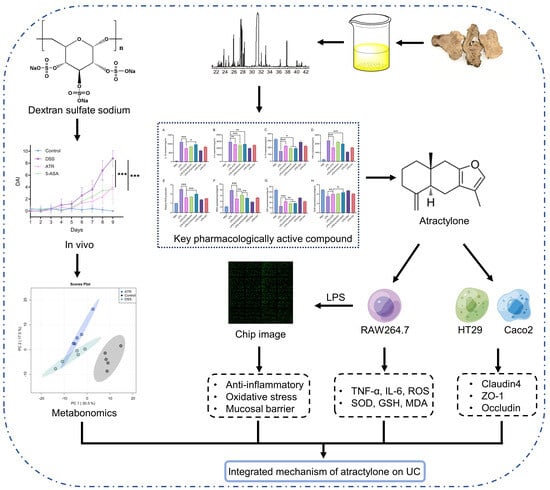
:1. Introduction
2. Results
2.1. Atractylone Is a Pivotal Pharmacologically Active Compound
2.2. ATR Inhibits LPS-Induced Inflammatory Reactions in RAW264.7 Cells
2.3. ATR Is Effective in Restoring the Intestinal Mucosal Barrier
2.4. Phosphorylation Antibody Chip Analysis
2.5. Experimental Validation of ATR’s Impact on UC In Vivo
2.6. Analysis of Potential Metabolites in Colon Tissue
3. Discussion
4. Materials and Methods
4.1. Preparation of AO and ATR
4.2. Effects of AO on Antioxidant Properties and Inflammatory Responses
4.3. ATR Inhibits Inflammatory and Oxidative Stress In Vitro
4.3.1. Cells
4.3.2. TNF-α and IL-6 Levels and Antioxidant Activity Assessment
4.3.3. Reactive Oxygen Species Measurements
4.3.4. Western Blotting
4.4. Analysis of Phosphospecific Protein Microarray
4.5. Establishment of the Ulcerative Colitis Model in Mice
4.6. GC-TOF-MS Conditions
4.7. Data Processing
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soroosh, A.; Fang, K.; Hoffman, J.M.; Law, I.K.M.; Videlock, E.; Lokhandwala, Z.A.; Zhao, J.J.; Hamidi, S.; Padua, D.M.; Frey, M.R.; et al. Loss of miR-24-3p promotes epithelial cell apoptosis and impairs the recovery from intestinal inflammation. Cell Death Dis. 2021, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Lemann, M. Review article: Remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol. Ther. 2011, 33, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Lin, T.W.; Huang, Y.L.; Huang, R.L. Induction of apoptosis and differentiation by atractylenolide-1 isolated from Atractylodes macrocephala in human leukemia cells. Bioorg. Med. Chem. Lett. 2016, 26, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.T.; Kao, K.T.; Weng, C.S. In vitro antibacterial and cytotoxic activities of plasma-modified polyethylene terephthalate nonwoven dressing with aqueous extract of Rhizome Atractylodes macrocephala. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-H.; Park, J.-M.; Jeong, M.; Han, Y.-M.; Go, E.-J.; Park, J.; Kim, H.; Han, J.G.; Kwon, O.; Hahm, K.B. Heme Oxygenase-1 Induction and Anti-inflammatory Actions of and Extracts Prevented Colitis and Was More Effective than Sulfasalazine in Preventing Relapse. Gut Liver 2017, 11, 655–666. [Google Scholar] [CrossRef]
- Wu, Y.X.; Lu, W.W.; Geng, Y.C.; Yu, C.H.; Sun, H.J.; Kim, Y.J.; Zhang, G.; Kim, T. Antioxidant, Antimicrobial and Anti-Inflammatory Activities of Essential Oil Derived from the Wild Rhizome of Atractylodes macrocephala. Chem. Biodivers. 2020, 17, e2000268. [Google Scholar] [CrossRef]
- Parikh, K.; Antanaviciute, A.; Fawkner-Corbett, D.; Jagielowicz, M.; Aulicino, A.; Lagerholm, C.; Davis, S.; Kinchen, J.; Chen, H.H.; Alham, N.K.; et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 2019, 567, 49–55. [Google Scholar] [CrossRef]
- Gu, S.; Li, L.; Huang, H.; Wang, B.; Zhang, T. Antitumor, Antiviral, and Anti-Inflammatory Efficacy of Essential Oils from Atractylodes macrocephala Koidz. Produced with Different Processing Methods. Molecules 2019, 24, 2956. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Z.; He, Y.; Gu, C.; Zhu, K.; Li, S.; Li, Y.; Lu, X.; Liu, J.; Zhang, N.; et al. Magnolol treatment attenuates dextran sulphate sodium-induced murine experimental colitis by regulating inflammation and mucosal damage. Life Sci. 2018, 196, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, H.; Tan, D.; Zhang, S.; Zhong, Z.; Wang, S.; Vong, C.T.; Wang, Y. A recent update on the use of Chinese medicine in the treatment of inflammatory bowel disease. Phytomedicine 2021, 92, 153709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Yue, Y.H.; Han, H.; Chen, X.L.; Lu, Y.G.; Zheng, J.M.; Hou, H.T.; Lang, X.M.; He, L.L.; Hu, Q.L.; et al. Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis. World J. Gastroenterol. 2017, 23, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Barnes, R.C.; Pfent, C.M.; Talcott, S.T.; Dashwood, R.H.; Mertens-Talcott, S.U. Mango polyphenolics reduce inflammation in intestinal colitis-involvement of the miR-126/PI3K/AKT/mTOR axis in vitro and in vivo. Mol. Carcinog. 2017, 56, 197–207. [Google Scholar] [CrossRef]
- Rahmani, F.; Asgharzadeh, F.; Avan, A.; Barneh, F.; Parizadeh, M.R.; Ferns, G.A.; Ryzhikov, M.; Ahmadian, M.R.; Giovannetti, E.; Jafari, M.; et al. Rigosertib potently protects against colitis-associated intestinal fibrosis and inflammation by regulating PI3K/AKT and NF-kappaB signaling pathways. Life Sci. 2020, 249, 117470. [Google Scholar] [CrossRef]
- Setia, S.; Nehru, B.; Sanyal, S.N. The PI3K/Akt pathway in colitis associated colon cancer and its chemoprevention with celecoxib, a Cox-2 selective inhibitor. Biomed. Pharmacother. 2014, 68, 721–727. [Google Scholar] [CrossRef]
- Imajo, M.; Tsuchiya, Y.; Nishida, E. Regulatory mechanisms and functions of MAP kinase signaling pathways. IUBMB Life 2006, 58, 312–317. [Google Scholar] [CrossRef]
- Chen, P.Y.; Yuan, C.; Hong, Z.C.; Zhang, Y.; Ke, X.G.; Yu, B.; Wang, C.; Xiao, X.C.; Wu, H.Z.; Yang, Y.F. Revealing the mechanism of “Huai Hua San” in the treatment of ulcerative colitis based on network pharmacology and experimental study. J. Ethnopharmacol. 2021, 281, 114321. [Google Scholar] [CrossRef]
- Katsanos, K.H.; Papadakis, K.A. Inflammatory Bowel Disease: Updates on Molecular Targets for Biologics. Gut Liver 2017, 11, 455–463. [Google Scholar] [CrossRef]
- Cohen, B.L.; Sachar, D.B. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ 2017, 357, j2505. [Google Scholar] [CrossRef]
- Lopez, A.; Ford, A.C.; Colombel, J.F.; Reinisch, W.; Sandborn, W.J.; Peyrin-Biroulet, L. Efficacy of tumour necrosis factor antagonists on remission, colectomy and hospitalisations in ulcerative colitis: Meta-analysis of placebo-controlled trials. Dig. Liver Dis. 2015, 47, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Dincer, Y.; Erzin, Y.; Himmetoglu, S.; Gunes, K.N.; Bal, K.; Akcay, T. Oxidative DNA damage and antioxidant activity in patients with inflammatory bowel disease. Dig Dis. Sci. 2007, 52, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Waetzig, G.H.; Seegert, D.; Rosenstiel, P.; Nikolaus, S.; Schreiber, S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J. Immunol. 2002, 168, 5342–5351. [Google Scholar] [CrossRef]
- Kucharzik, T.; Walsh, S.V.; Chen, J.; Parkos, C.A.; Nusrat, A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am. J. Pathol. 2001, 159, 2001–2009. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Liao, J.; Seril, D.N.; Yang, A.L.; Lu, G.G.; Yang, G.Y. Inhibition of chronic ulcerative colitis associated adenocarcinoma development in mice by inositol compounds. Carcinogenesis 2007, 28, 446–454. [Google Scholar] [CrossRef]
- Bradford, E.M.; Thompson, C.A.; Goretsky, T.; Yang, G.Y.; Rodriguez, L.M.; Li, L.; Barrett, T.A. Myo-inositol reduces beta-catenin activation in colitis. World J. Gastroenterol. 2017, 23, 5115–5126. [Google Scholar] [CrossRef]
- Xie, D.; Li, F.; Pang, D.; Zhao, S.; Zhang, M.; Ren, Z.; Geng, C.; Wang, C.; Wei, N.; Jiang, P. Systematic Metabolic Profiling of Mice with Dextran Sulfate Sodium-Induced Colitis. J. Inflamm. Res. 2021, 14, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Hu, C.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- McWhirt, J.; Sathyanesan, M.; Sampath, D.; Newton, S.S. Effects of restraint stress on the regulation of hippocampal glutamate receptor and inflammation genes in female C57BL/6 and BALB/c mice. Neurobiol. Stress 2019, 10, 100169. [Google Scholar] [CrossRef]
- King, S.; Jelen, L.A.; Horne, C.M.; Cleare, A.; Pariante, C.M.; Young, A.H.; Stone, J.M. Inflammation, Glutamate, and Cognition in Bipolar Disorder Type II: A Proof of Concept Study. Front. Psychiatry 2019, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Bondy, S.C. Increased oxidative stress, inflammation, and glutamate: Potential preventive and therapeutic targets for hearing disorders. Mech. Ageing Dev. 2020, 185, 111191. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, J.F.; Fei, S.J.; Zhu, S.P.; Zhu, J.Z.; Qiao, X.; Liu, Z.B. Glutamate microinjection into the hypothalamic paraventricular nucleus attenuates ulcerative colitis in rats. Acta Pharmacol. Sin. 2014, 35, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Hikino, Y.; Yosioka, I. Studies on the Constituents of Atractylodes. Ix. Structure and Autoxidation of Atractylon. Chem. Pharm. Bull. 1964, 12, 755–760. [Google Scholar] [CrossRef]
- Li, L.; Dou, D. Study on Stability of Atractylon in Volatile Oil of Atractylodes Macrocephala Koidz. World Sci. Technol. Mod. Tradit. Chin. Med. 2014, 16, 193–198. [Google Scholar]
- Kringel, D.H.; Antunes, M.D.; Klein, B.; Crizel, R.L.; Wagner, R.; de Oliveira, R.P.; Dias, A.R.G.; Zavareze, E.D.R. Production, Characterization, and Stability of Orange or Eucalyptus Essential Oil/beta-Cyclodextrin Inclusion Complex. J. Food Sci. 2017, 82, 2598–2605. [Google Scholar] [CrossRef]
- Tang, X.; Yang, M.; Gu, Y.; Jiang, L.; Du, Y.; Liu, J. Orally Deliverable Dual-Targeted Pellets for the Synergistic Treatment of Ulcerative Colitis. Drug Des. Dev. Ther. 2021, 15, 4105–4123. [Google Scholar] [CrossRef]
- Shi, W.; Yan, R.; Huang, L. Preparation and insecticidal performance of sustained-release cinnamon essential oil microemulsion. J. Sci. Food Agric. 2022, 102, 1397–1404. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; He, Y.; Wang, N.; Li, Y.; Du, Y.; He, N.; Wang, B.; Zhang, T. Atractylone in the Atractylodes macrocephala Rhizoma Essential Oil and Its Anti-Inflammatory Activity. Molecules 2023, 28, 7340. https://doi.org/10.3390/molecules28217340
Li L, He Y, Wang N, Li Y, Du Y, He N, Wang B, Zhang T. Atractylone in the Atractylodes macrocephala Rhizoma Essential Oil and Its Anti-Inflammatory Activity. Molecules. 2023; 28(21):7340. https://doi.org/10.3390/molecules28217340
Chicago/Turabian StyleLi, Ling, Yihao He, Nan Wang, Yuting Li, Yaoyao Du, Ning He, Bing Wang, and Tong Zhang. 2023. "Atractylone in the Atractylodes macrocephala Rhizoma Essential Oil and Its Anti-Inflammatory Activity" Molecules 28, no. 21: 7340. https://doi.org/10.3390/molecules28217340