Nano-Zero-Valent Zinc-Modified Municipal Sludge Biochar for Phosphorus Removal
Abstract
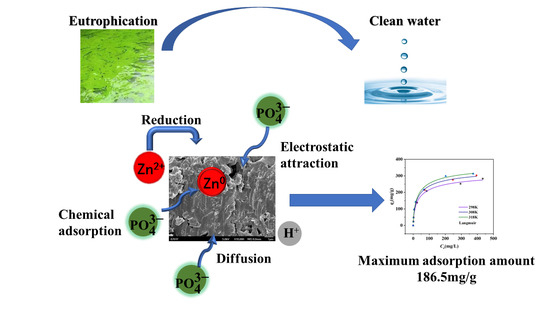
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Surface Structure of the Biochar
2.2. Adsorption Kinetics Study
2.3. Adsorption Thermodynamics Study
2.4. Effect of pH on Phosphorus Adsorption Capacity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Biochar Preparation
3.3. Biochar Characterization
3.4. Batch Adsorption Experiments
3.4.1. Adsorption Kinetics Experiment
3.4.2. Isothermal Adsorption Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-friendly montmorillonite-biochar composites: Facile production and tunable adsorption-release of ammonium and phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- Hu, W.R.; Xie, Y.; Lu, S.; Li, F.Y.; Xie, T.H.; Zhang, Y.K.; Wang, Y.B. One-step synthesis of nitrogen-doped sludge carbon as a bifunctional material for the adsorption and catalytic oxidation of organic pollutants. Sci. Total. Environ. 2019, 680, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wang, X.B.; Yu, S.L.; Deng, S.H.; Tan, H.Z.; Mikulčić, H. Process design and optimization on self-sustaining pyrolysis and carbonization of municipal sewage sludge. Waste Manag. 2023, 159, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.A.E.H.; N’diaye A., D.; Benahdach, K.; Ahrouch, M.; Lahcen, A.A.; Silanpaa, M.; Stitou, M. From disposal problem to valuable product: The route of sewage sludge as an adsorbent for congo red removal. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Jo, J.Y.; Choi, J.H.; Tsang, Y.F. Pelletized adsorbent of alum sludge and bentonite for removal of arsenic. Env. Pollut. 2021, 277, 116747. [Google Scholar] [CrossRef]
- Kumar, R.; Kang, C.U.; Mohan, D.; Khan, M.A.; Lee, J.; Lee, S.; Jeon, B. Waste sludge derived adsorbents for arsenate removal from water. Chemosphere 2020, 239, 124832. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Mckay, G.; Kochkodan, V.; Atieh, M.A.; Tareq, A. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X.D. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Keifer, K.; Bai, Y.; Kurt, K.; Daniel, A.; Kenney, J. Ciprofloxacin adsorption on sewage sludge derived biochar. Geol. Soc. Am. Abstr. Programs 2022, 54, 5. [Google Scholar]
- Ahmed, M.; Heba, H.E.; Amr, A.; Menem, N.; Raynaud, P.; Moustafa, Y.M.; Elsayed, M.A.; Nada, A.A. Efficiently activated carbons from corn cob for methylene blue adsorption. Appl. Surf. Sci. Adv. 2021, 3, 123–135. [Google Scholar]
- Pouran, P.; Ali, T.; Mohsen, T.; Ghaedi, M.; Haghdoust, S. Chapter 1-Fundamentals of adsorption technology. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 1–70. [Google Scholar]
- Pouran, P.; Mohsen, T.; Ali, T.; Ghaedi, M. Chapter 2-Adsorbent. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 71–210. [Google Scholar]
- Hou, Q.J.; Meng, P.P.; Pei, H.Y.; Hu, W.R.; Chen, Y. Phosphorus adsorption characteristics of alum sludge: Adsorption capacity and the forms of phosphorus retained in alum sludge. Mater. Lett. 2018, 229, 31–35. [Google Scholar] [CrossRef]
- Gillingham, M.; Gomes, R.; Ferrari, R.; West, H.M. Sorption, separation and recycling of ammonium in agricultural soils: A viable application for magnetic biochar. Sci. Total. Env. 2022, 812, 151440. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Samia, A.K.; Nouf, A.A.; Owija, N.Y. Removal of Acid Red dye from aqueous solution using zero-valent copper and zero-valent zinc nanoparticles. Desalination Water Treat. 2019, 141, 310–320. [Google Scholar]
- Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X.K. Efficient elimination of organic and inorganic pollutants by biochar and biochar-based materials. Biochar 2020, 2, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bai, X.; Ye, Z. Recent progress in heavy metal ion decontamination based on metal-organic frameworks. Nanomaterials 2020, 10, 1481. [Google Scholar] [CrossRef]
- Kavitha, B.; Reddy, P.V.L.; Kim, B.; Lee, S.S.; Pandey, S.K.; Kim, K. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manag. 2018, 227, 146–154. [Google Scholar] [CrossRef]
- Wang, M.J.; Hu, S.K.; Wang, Q.G.; Liang, Y.; Liu, C.R.; Xu, H.; Ye, Q. Enhanced nitrogen and phosphorus adsorption performance and stabilization by novel panda manure biochar modified by CMC stabilized nZVZ composite in aqueous solution: Mechanisms and application potential. J. Clean. Prod. 2021, 291, 125221. [Google Scholar] [CrossRef]
- Zhao, H.L.; Wu, S.; Liu, C.Y.; Yan, X.T.; Xu, X.; Fu, S.S.; Wang, Y.B.; Su, Q.; Wang, X.; Yang, Q.L. Encapsulation Fe-Nx combined with Co@C to construct efficient oxygen reduction catalysts with bimetallic sites and the application of Zn-air batteries. Mater. Today Chem. 2022, 26, 101174. [Google Scholar] [CrossRef]
- Lise, B.; Stephen, J.; Margaret, G.; Donne, S.; Munroe, P.; Marjo, C.E. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater. Ecol. Eng. 2019, 142, 100005. [Google Scholar]
- Gao, C.; Fan, J.; Zhang, X.J.; Gong, Z.W.; Tan, Z.Y. Sediment metals adhering to biochar enhanced phosphorus adsorption in sediment capping. Water Sci. Technol. 2021, 84, 2057–2067. [Google Scholar] [CrossRef]
- Anyu, L.; Hua, D.; Yuqing, W.; Ye, C.H.; Jiang, Y.H. Strong Adsorption of Phosphorus by ZnAl-LDO-Activated Banana Biochar: An Analysis of Adsorption Efficiency, Thermodynamics and Internal Mechanisms. ACS Omega 2021, 6, 7402–7412. [Google Scholar]
- Li, A.; Deng, H.; Ye, C.; Jiang, Y.H. Fabrication and characterization of novel ZnAl-layered double hydroxide for the superadsorption of organic contaminants from wastewater. ACS Omega 2020, 5, 15152–15161. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, M.; Hu, H.; Zhang, Q.W.; Tao, D.P. Mechanochemical synthesis of novel Pt modified ZnAl-LDH for effective ciprofloxacin photodegradation. Solid. State Chem. 2020, 290, 121594. [Google Scholar] [CrossRef]
- Xian, Y.; Zhang, X.L.; Lei, Y. Influencing factors for phosphorus removal by modified bio-ceramic substrates coated with ZnAl-LDHs synthesized by different modification conditions. Huanjing Kexue 2018, 39, 2184–2194. [Google Scholar]
- Verma, R.; Suthar, S. Performance assessment of horizontal and vertical surface flow constructed wetland system in wastewater treatment using multivariate principal component analysis. Ecol. Eng. 2018, 116, 121–126. [Google Scholar] [CrossRef]
- Zhou, B.; Wei, X.; Wang, Y.; Huang, Q.Y.; Hong, B.; Wei, Y.Z. Effect of lanthanum addition on microstructures and corrosion behavior of ZnAl-LDHs film of 6061 aluminum alloys. Surf. Coat. Technol. 2019, 379, 125056. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Xu, D.H.; Hao, B.T.; Liu, L.; Wang, S.Z.; Wu, Z.Q. Thermochemical methods for the treatment of municipal sludge. J. Clean. Prod. 2021, 331, 127811. [Google Scholar] [CrossRef]
- Peyman, G.; Alireza, K.; Reza, D.C.S.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@biochar nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar]
- Jung, K.W.; Lee, S.Y.; Lee, Y.J. Facile one-pot hydrothermal synthesis of cubic spinel-type manganese ferrite/biochar composites for environmental remediation of heavy metals from aqueous solutions. Bioresour. Technol. 2018, 261, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Meng, Y.; Su, J.H.; Wang, X.J. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci. Total. Environ. 2017, 603–604, 39–48. [Google Scholar] [CrossRef]
- Ruzickova, J.; Koval, S.; Raclavska, H.; Kucbel, M.; Svedova, B.; Raclavsky, K.; Juchelkova, D.; Scala, F. A comprehensive assessment of potential hazard caused by organic compounds in biochar for agricultural use. J. Hazard. Mater. 2021, 403, 123644. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, Y.; He, Q.; Zhao, D.; Zhang, K.Q.; Shen, S.Z.; Wang, F. Performance and mechanism of a biochar-based Ca-La composite for the adsorption of phosphate from water. J. Environ. Chem. Eng. 2021, 9, 105267. [Google Scholar] [CrossRef]
- Aghaei, H.; Ghavi, M.; Hashemkhani, G.; Keshavarz, M. Utilization of two modified layered doubled hydroxides as supports for immobilization of Candida rugosa lipase. Int. J. Biol. Macromol. 2020, 162, 74–83. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Wang, H.; Xue, J.B.; Lv, Q.; Pang, G.B. Efficient recovery of phosphate from aqueous solution using biochar derived from co-pyrolysis of sewage sludge with eggshell. J. Environ. Chem. Eng. 2021, 9, 105354. [Google Scholar] [CrossRef]
- He, L.; Zhang, W.; Zhang, X.; Bai, X.; Chen, J.K.; Ikram, M.; Zhang, G.; Shi, K.Y. 3D flower-like NiCo-LDH composites for a high-performance NO2 gas sensor at room temperature. Colloids Surf. A 2020, 603, 125142. [Google Scholar] [CrossRef]








| Material | MSBC | nZVZ–MSBC | nZVZ–MSBC (After Adsorption) |
|---|---|---|---|
| SBET (m2/g) | 71.24 | 162.77 | 166.99 |
| Dpore (nm) | 14.30 | 15.05 | 7.21 |
| Vpore (cm3/g) | 0.25 | 0.61 | 0.30 |
| Material | nZVZ–MSBC | ||
|---|---|---|---|
| T (K) | 298 | 308 | 318 |
| qe a | 167.77 | 177.04 | 186.51 |
| Pseudo-first-order | |||
| qe b (mg/g) | 156.42 | 165.94 | 176.37 |
| k1 (min) | 0.01185 | 0.01859 | 0.02965 |
| R2 | 0.9704 | 0.9613 | 0.97161 |
| Pseudo-second-order | |||
| qe b (mg/g) | 172.55 | 179.88 | 189.16 |
| k2 g/(mg·min) | 9.29 × 10−5 | 1.52 × 10−4 | 2.43 × 10−4 |
| R2 | 0.99293 | 0.99061 | 0.99581 |
| T (K) | 298 | 308 | 318 |
|---|---|---|---|
| Langmuir | |||
| KL (L/mg) | 0.0901 | 0.0924 | 0.0896 |
| Qm (mg/g) | 328.51 | 354.67 | 376.41 |
| R2 | 0.99045 | 0.99465 | 0.99237 |
| Freundlich | |||
| KF (mg/g(L/mg)1/n) | 48.395 | 51.800 | 53.770 |
| 1/n | 0.03299 | 0.03164 | 0.03478 |
| R2 | 0.9684 | 0.97186 | 0.96727 |
| Sips | |||
| Qm (mg/g) | 328.58 | 354.75 | 376.54 |
| m | 0.6649 | 0.67283 | 0.6826 |
| KS (L/mg) | 0.02679 | 0.02902 | 0.02921 |
| R2 | 0.99279 | 0.99589 | 0.99407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, W.; Zhang, H.; He, D. Nano-Zero-Valent Zinc-Modified Municipal Sludge Biochar for Phosphorus Removal. Molecules 2023, 28, 3231. https://doi.org/10.3390/molecules28073231
Zhang Y, Zhang W, Zhang H, He D. Nano-Zero-Valent Zinc-Modified Municipal Sludge Biochar for Phosphorus Removal. Molecules. 2023; 28(7):3231. https://doi.org/10.3390/molecules28073231
Chicago/Turabian StyleZhang, Yupeng, Wenbo Zhang, Hong Zhang, and Dandan He. 2023. "Nano-Zero-Valent Zinc-Modified Municipal Sludge Biochar for Phosphorus Removal" Molecules 28, no. 7: 3231. https://doi.org/10.3390/molecules28073231