Photochemical Implications of Changes in the Spectral Properties of Chromophoric Dissolved Organic Matter: A Model Assessment for Surface Waters
Abstract
:1. Introduction
2. Results and Discussion
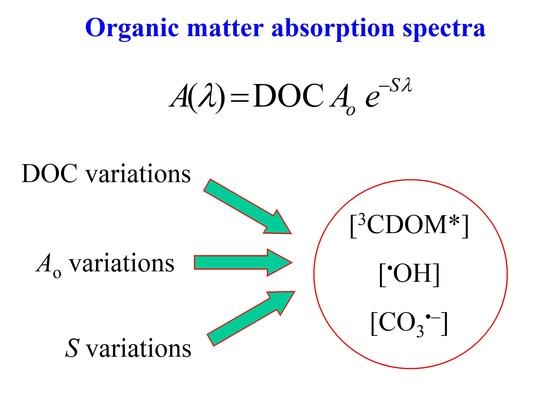
2.1. Effect of CDOM Spectral Features on the Steady-State [3CDOM*]
2.2. Effect of CDOM Spectral Features on the Steady-State [•OH] and [CO3•−]
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Remucal, C.K. The role of indirect photochemical degradation in the environmental fate of pesticides: A review. Environ. Sci.-Process. Impacts 2014, 16, 628–653. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Minella, M.; Maurino, V.; Minero, C. Indirect photochemistry in sunlit surface waters: Photoinduced production of reactive transient species. Chem.-Eur. J. 2014, 20, 10590–10606. [Google Scholar] [CrossRef] [PubMed]
- Young, R.B.; Latch, D.E.; Mawhinney, D.B.; Nguyen, T.-H.; Davis, J.C.C.; Borch, T. Direct photodegradation of androstenedione and testosterone in natural sunlight: Inhibition by dissolved organic matter and reduction of endocrine disrupting potential. Environ. Sci. Technol. 2013, 47, 8416–8424. [Google Scholar] [CrossRef]
- Prasse, C.; Wenk, J.; Jasper, J.T.; Ternes, T.A.; Sedlak, D.L. Co-occurrence of photochemical and microbiological transformation processes in open-water unit process wetlands. Environ. Sci. Technol. 2015, 49, 14136–14145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef]
- Gao, P.; Ding, Y.; Li, H.; Xagoraraki, I. Occurrence of pharmaceuticals in a municipal wastewater treatment plant: Mass balance and removal processes. Chemosphere 2012, 88, 17–24. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2014, 86, 2813–2848. [Google Scholar] [CrossRef]
- Knopp, G.; Prasse, C.; Ternes, T.A.; Cornel, P. Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Res. 2016, 100, 580–592. [Google Scholar] [CrossRef]
- Fang, H.; Ling, Z.; Guan, F.; Liao, W.; Lai, F.; Liang, X. Photophysical and photochemical insights into the photodegradation of tricyclazole and pymetrozine in water bodies of a rice field. Environ. Chem. 2020, 17, 436–444. [Google Scholar] [CrossRef]
- Liang, X.; Guan, F.; Ling, Z.; Wang, H.; Tao, Y.; Kraka, E.; Huang, H.; Yu, C.; Li, D.; He, J.; et al. Pivotal role of water molecules in the photodegradation of pymetrozine: New insights for developing green pesticides. J. Hazard. Mater. 2022, 423 (Pt B), 127197. [Google Scholar] [CrossRef]
- Vione, D.; Scozzaro, A. Photochemistry of surface fresh waters in the framework of climate change. Environ. Sci. Technol. 2019, 53, 7945–7963. [Google Scholar] [CrossRef] [PubMed]
- Vione, D. Photochemical reactions and the self-depuration of sunlit freshwaters. Environ. Res. Ecol. 2023, 2, 012001. [Google Scholar] [CrossRef]
- Rosario-Ortiz, F.L.; Canonica, S. Probe compounds to assess the photochemical activity of dissolved organic matter. Environ. Sci. Technol. 2016, 50, 12532–12547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mc Neill, K.; Canonica, S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ. Sci.-Process. Impacts 2016, 18, 1381–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossola, R.; Jönsson, O.M.; Moor, K.; McNeill, K. Singlet oxygen quantum yields in environmental waters. Chem. Rev. 2021, 121, 4100–4146. [Google Scholar] [CrossRef]
- Loiselle, S.; Vione, D.; Minero, C.; Maurino, V.; Tognazzi, A.; Dattilo, A.M.; Rossi, C.; Bracchini, L. Chemical and optical phototransformation of dissolved organic matter. Water Res. 2012, 46, 3197–3207. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, S.; Lian, L.; Song, W. Triplet-state photochemistry of dissolved organic matter: Triplet-state energy distribution and surface electric charge conditions. Environ. Sci. Technol. 2019, 53, 2482–2490. [Google Scholar] [CrossRef]
- Sha, H.; Yan, S.; Deng, Y.; Song, W. Photosensitized transformation of hydrogen peroxide in dissolved organic matter solutions under simulated solar irradiation. Environ. Sci. Technol. 2022, 56, 14080–14090. [Google Scholar] [CrossRef]
- Galgani, L.; Tognazzi, A.; Rossi, C.; Ricci, M.; Angel Galvez, J.; Dattilo, A.M.; Cozar, A.; Bracchini, L.; Loiselle, S.A. Assessing the optical changes in dissolved organic matter in humic lakes by spectral slope distributions. J. Photochem. Photobiol. B Biol. 2011, 102, 132–139. [Google Scholar] [CrossRef]
- Solomon, C.T.; Jones, S.E.; Weidel, B.C.; Buffam, I.; Fork, M.L.; Karlsson, J.; Larsen, S.; Lennon, J.T.; Read, J.S.; Sadro, S.; et al. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 2015, 18, 376–389. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Müller, R.A.; Norman, M.; Tranvik, L.J. Sensitivity of freshwaters to browning in response to future climate change. Clim. Chang. 2016, 134, 225–239. [Google Scholar] [CrossRef]
- Sobek, S.; Tranvik, L.J.; Prairie, Y.T.; Kortelainen, P.; Cole, J.J. Patterns and regulation of dissolved organic carbon: An analysis of 7500 widely distributed lakes. Limnol. Oceanogr. 2007, 58, 1208–1219. [Google Scholar] [CrossRef] [Green Version]
- Williamson, C.E.; Overholt, E.P.; Pilla, R.M.; Leach, T.H.; Brentrup, J.A.; Knoll, L.B.; Mette, E.M.; Moeller, R.E. Ecological consequences of long-term browning in lakes. Sci. Rep. 2015, 5, 18666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futter, M.N.; Starr, M.; Forsius, M.; Holmberg, M. Modelling the effects of climate on long-term patterns of dissolved organic carbon concentrations in the surface waters of a boreal catchment. Hydrol. Earth Syst. Sci. 2008, 12, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Koehler, B.; Barsotti, F.; Minella, M.; Landelius, T.; Minero, C.; Tranvik, L.J.; Vione, D. Simulation of photoreactive transients and of photochemical transformation of organic pollutants in sunlit boreal lakes across 14 degrees of latitude: A photochemical mapping of Sweden. Water Res. 2018, 129, 94–104. [Google Scholar] [CrossRef]
- Sperotto, A.; Molina, J.L.; Torresan, S.; Critto, A.; Pulido-Velazquez, M.; Marcomini, A. Water quality sustainability evaluation under uncertainty: A multi-scenario analysis based on Bayesian networks. Sustainability 2019, 11, 4764. [Google Scholar] [CrossRef] [Green Version]
- Jane, S.F.; Winslow, L.A.; Remucal, C.K.; Rose, K.C. Long-term trends and synchrony in dissolved organic matter characteristics in Wisconsin, USA, lakes: Quality, not quantity, is highly sensitive to climate. J. Geophys. Res. Biogeosci. 2017, 122, 546–561. [Google Scholar] [CrossRef]
- Calderaro, F.; Vione, D. Possible effect of climate change on surface-water photochemistry: A model assessment of the impact of browning on the photodegradation of pollutants in lakes during summer stratification. Epilimnion vs. whole-lake phototransformation. Molecules 2020, 25, 2795. [Google Scholar] [CrossRef]
- Bracchini, L.; Loiselle, S.; Dattilo, A.M.; Mazzuoli, S.; Cozar, A.; Rossi, C. The spatial distribution of optical properties in the ultraviolet and visible in an aquatic ecosystem. Photochem. Photobiol. 2004, 80, 139–149. [Google Scholar] [CrossRef]
- Vione, D. A critical view of the application of the APEX software (Aqueous Photochemistry of Environmentally-occurring Xenobiotics) to predict photoreaction kinetics in surface freshwaters. Molecules 2020, 25, 9. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Vione, D. The modelling of surface-water photoreactions made easier: Introducing the concept of ‘equivalent monochromatic wavelengths’. Water Res. 2021, 190, 116675. [Google Scholar] [CrossRef] [PubMed]
- Minero, C.; Chiron, S.; Falletti, G.; Maurino, V.; Pelizzetti, E.; Ajassa, R.; Carlotti, M.E.; Vione, D. Photochemical processes involving nitrite in surface water samples. Aquat. Sci. 2007, 69, 71–85. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Maberly, S.C.; Pitt, J.-A.; Davies, P.S.; Carvalho, L. Nitrogen and phosphorus limitation and the management of small productive lakes. Inland Waters 2020, 10, 159–172. [Google Scholar] [CrossRef] [Green Version]
- Lasagna, M.; De Luca, D.A.; Franchino, E. Nitrate contamination of groundwater in the western Po Plain (Italy): The effects of groundwater and surface water interactions. Environ. Earth Sci. 2016, 75, 240. [Google Scholar] [CrossRef]
- Da Silva, M.P.; De Carvalho, L.A.S.; Novo, E.; Jorge, D.S.F.; Barbosa, C.C.F. Use of optical absorption indices to assess seasonal variability of dissolved organic matter in Amazon floodplain lakes. Biogeosciences 2020, 17, 5355–5364. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Osburn, C.L.; Wang, M.; Qin, B.; Zhou, Y. Photobleaching response of different sources of chromophoric dissolved organic matter exposed to natural solar radiation using absorption and excitation-emission matrix spectra. PLoS ONE 2013, 8, e77515. [Google Scholar]
- Silva, M.P.; Mostafa, S.; McKay, G.; Rosario-Ortiz, F.L.; Teixeira, A.C.S.C. Photochemical fate of amicarbazone in aqueous media: Laboratory measurement and simulations. Environ. Engin. Sci. 2015, 32, 730–740. [Google Scholar] [CrossRef]
- Vione, D. Insights into the time evolution of slowly photodegrading contaminants. Molecules 2021, 26, 5223. [Google Scholar] [CrossRef]
- Yan, S.; Liu, Y.; Lian, L.; Li, R.; Ma, J.; Zhou, H.; Song, W. Photochemical formation of carbonate radical and its reaction with dissolved organic matters. Water Res. 2019, 161, 288–296. [Google Scholar] [CrossRef]
- Del Vecchio, R.; Blough, N.V. On the origin of the optical properties of humic substances. Environ. Sci. Technol. 2004, 38, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Carena, L.; Minella, M.; Barsotti, F.; Brigante, M.; Milan, M.; Ferrero, A.; Berto, S.; Minero, C.; Vione, D. Phototransformation of the herbicide propanil in paddy field water. Environ. Sci. Technol. 2017, 51, 2695–2704. [Google Scholar] [CrossRef]
- Canonica, S. Oxidation of aquatic organic contaminants induced by excited triplet states. Chimia 2007, 61, 641–644. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, T.; Zeng, T.; Rosario-Ortiz, F.L. Photodegradation of cyanotoxins in surface waters. Water Res. 2021, 192, 116804. [Google Scholar] [CrossRef]
- Vione, D.; Rosario-Ortiz, F.L. Foreseen effects of climate-impacted scenarios on the photochemical fate of selected cyanotoxins in surface freshwaters. Environ. Sci. Technol. 2021, 55, 10928–10934. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, P.W.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, G.; Minella, M.; Maurino, V.; Minero, C.; Vione, D. Photochemical transformation of atrazine and formation of photointermediates under conditions relevant to sunlit surface waters: Laboratory measures and modelling. Water Res. 2013, 47, 6211–6222. [Google Scholar] [CrossRef]
- De Laurentiis, E.; Chiron, S.; Kouras-Hadef, S.; Richard, C.; Minella, M.; Maurino, V.; Minero, C.; Vione, D. Photochemical fate of carbamazepine in surface freshwaters: Laboratory measures and modeling. Environ. Sci. Technol. 2012, 46, 8164–8173. [Google Scholar] [CrossRef] [Green Version]
- Minella, M.; Giannakis, S.; Mazzavillani, A.; Maurino, V.; Minero, C.; Vione, D. Phototransformation of Acesulfame K in surface waters: Comparison of two techniques for the measurement of the second-order rate constants of indirect photodegradation, and modelling of photoreaction kinetics. Chemosphere 2017, 186, 185–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author (s) and contributor (s) and not of MDPI and/or the editor (s). MDPI and/or the editor (s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altare, N.; Vione, D. Photochemical Implications of Changes in the Spectral Properties of Chromophoric Dissolved Organic Matter: A Model Assessment for Surface Waters. Molecules 2023, 28, 2664. https://doi.org/10.3390/molecules28062664
Altare N, Vione D. Photochemical Implications of Changes in the Spectral Properties of Chromophoric Dissolved Organic Matter: A Model Assessment for Surface Waters. Molecules. 2023; 28(6):2664. https://doi.org/10.3390/molecules28062664
Chicago/Turabian StyleAltare, Nicole, and Davide Vione. 2023. "Photochemical Implications of Changes in the Spectral Properties of Chromophoric Dissolved Organic Matter: A Model Assessment for Surface Waters" Molecules 28, no. 6: 2664. https://doi.org/10.3390/molecules28062664