α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity
Abstract
:1. Introduction
2. Results
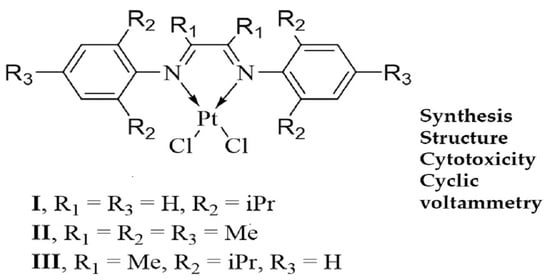
2.1. Synthesis and Characterization of [(dpp-DAD)PtCl2] (I), [(Mes-DAD(Me)2)PtCl2] (II) and [(dpp-DAD(Me)2)PtCl2] (III)
2.2. Molecular Structures of I and II
2.3. Cyclic Voltammetry of I–III
2.4. Biological Activity of I–III
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of [(dpp-DAD)PtCl2] (I)
3.3. Synthesis of [(Mes-DAD(Me)2)PtCl2] (II)
3.4. Synthesis of [(dpp-DAD(Me)2)PtCl2] (III)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hogarth, G. Metal-Dithiocarbamate Complexes: Chemistry and Biological Activity. Mini-Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Sadler, P.J. Current Applications and Future Potential for Bioinorganic Chemistry in the Development of Anticancer Drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1, 10-Phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooley, M.E.; Davis, L.; Abrahm, J. Cisplatin: A Clinical Review. Part II—Nursing Assessment and Management of Side Effects of Cisplatin. Cancer Nurs. 1994, 17, 283–293. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular Mechanisms of Cisplatin Resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems Biology of Cisplatin Resistance: Past, Present and Future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedushkin, I.L.; Skatova, A.A.; Chudakova, V.A.; Fukin, G.K. Four-Step Reduction of Dpp-Bian with Sodium Metal: Crystal Structures of the Sodium Salts of the Mono-, Di-, Tri- and Tetraanions of Dpp-Bian. Angew. Chem. Int. Ed. 2003, 42, 3294–3298. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-Innocent Ligands in Bioinorganic Chemistry—An Overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef] [Green Version]
- Kaim, W.; Schwederski, B. Cooperation of Metals with Electroactive Ligands of Biochemical Relevance: Beyond Metalloporphyrins. Pure Appl. Chem. 2004, 76, 351–364. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, M.; Zhang, Y.; Fang, F.; Li, M.; An, F.; Zhao, D.; Zhang, J. Free Radical as a Double-Edged Sword in Disease: Deriving Strategic Opportunities for Nanotherapeutics. Coord. Chem. Rev. 2023, 475, 214875. [Google Scholar] [CrossRef]
- Van Koten, G.; Vrieze, K. 1,4-Diaza-1,3-Butadiene (α-Diimine) Ligands: Their Coordination Modes and the Reactivity of Their Metal Complexes. In Advances in Organometallic Chemistry; Academic Press: Cambridge, MA, USA, 1982; Volume 21, pp. 151–239. ISBN 0065-3055. [Google Scholar]
- Nikolaevskaya, E.N.; Druzhkov, N.O.; Syroeshkin, M.A.; Egorov, M.P. Chemistry of Diazadiene Type Ligands with Extra Coordination Groups. Prospects of Reactivity. Coord. Chem. Rev. 2020, 417, 213353. [Google Scholar] [CrossRef]
- Hill, N.J.; Vargas-Baca, I.; Cowley, A.H. Recent Developments in the Coordination Chemistry of Bis(Imino)Acenaphthene (BIAN) Ligands with s- and p-Block Elements. Dalton Trans. 2009, 240–253. [Google Scholar] [CrossRef]
- Mealli, C.; Ienco, A.; Phillips, A.D.; Galindo, A. A Critical Review of Electronic Effects in Enediamido and α-Diimino Complexes of the Group 4 Metals. Eur. J. Inorg. Chem. 2007, 2007, 2556–2568. [Google Scholar] [CrossRef]
- Sandl, S.; Maier, T.M.; van Leest, N.P.; Kröncke, S.; Chakraborty, U.; Demeshko, S.; Koszinowski, K.; de Bruin, B.; Meyer, F.; Bodensteiner, M.; et al. Cobalt-Catalyzed Hydrogenations via Olefin Cobaltate and Hydride Intermediates. ACS Catal. 2019, 9, 7596–7606. [Google Scholar] [CrossRef]
- Bart, S.C.; Hawrelak, E.J.; Lobkovsky, E.; Chirik, P.J. Low-Valent α-Diimine Iron Complexes for Catalytic Olefin Hydrogenation. Organometallics 2005, 24, 5518–5527. [Google Scholar] [CrossRef]
- Crossetti, G.L.; Dias, M.L.; Queiroz, B.T.; Silva, L.P.; Ziglio, C.M.; Bomfim, J.A.S.; Filgueiras, C.A.L. Ethylene Polymerization with Imine and Phosphine Nickel Complexes Containing Isothiocyanate. Appl. Organomet. Chem. 2004, 18, 331–336. [Google Scholar] [CrossRef]
- Lima, G.; Nunes, E.; Dantas, R.; Simone, C.; Meneghetti, M.; Meneghetti, S. Catalytic Behaviors of CoII and MnII Compounds Bearing α-Diimine Ligands for Oxidative Polymerization or Drying Oils. J. Braz. Chem. Soc. 2018, 29, 412–418. [Google Scholar] [CrossRef]
- Palmer, W.N.; Diao, T.; Pappas, I.; Chirik, P.J. High-Activity Cobalt Catalysts for Alkene Hydroboration with Electronically Responsive Terpyridine and α-Diimine Ligands. ACS Catal. 2015, 5, 622–626. [Google Scholar] [CrossRef]
- Moskalev, M.V.; Skatova, A.A.; Razborov, D.A.; Bazanov, A.A.; Bazyakina, N.L.; Sokolov, V.G.; Fedushkin, I.L. Magnesium and Calcium Complexes of ArBIG-Bian and Their Reactivity towards CO2 (ArBIG-Bian=1,2-Bis[(2,6-Dibenzhydryl-4-Methylphenyl)Imino]Acenaphthene). Eur. J. Inorg. Chem. 2021, 2021, 1890–1896. [Google Scholar] [CrossRef]
- Koptseva, T.S.; Sokolov, V.G.; Ketkov, S.Y.; Rychagova, E.A.; Cherkasov, A.V.; Skatova, A.A.; Fedushkin, I.L. Reversible Addition of Carbon Dioxide to Main Group Metal Complexes at Temperatures about 0 °C. Chem.—A Eur. J. 2021, 27, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Skatova, A.A.; Cherkasov, A.V.; Fedushkin, I.L. Addition of Phenylacetylene and Camphor to the Complex [(Dpp-Bian)Eu(Dme)2] (Dpp-Bian Is the 1,2-Bis[(2,6-Diisopropylphenyl)Imino]Acenaphthene Dianion). Russ. Chem. Bull. 2017, 66, 1187–1195. [Google Scholar] [CrossRef]
- Wang, P.; Saber, M.R.; VanNatta, P.E.; Yap, G.P.A.; Popescu, C.V.; Scarborough, C.C.; Kieber-Emmons, M.T.; Dunbar, K.R.; Riordan, C.G. Molecular and Electronic Structures and Single-Molecule Magnet Behavior of Tris(Thioether)–Iron Complexes Containing Redox-Active α-Diimine Ligands. Inorg. Chem. 2021, 60, 6480–6491. [Google Scholar] [CrossRef]
- Khusniyarov, M.M.; Weyhermüller, T.; Bill, E.; Wieghardt, K. Reversible Electron Transfer Coupled to Spin Crossover in an Iron Coordination Salt in the Solid State. Angew. Chem. Int. Ed. 2008, 47, 1228–1231. [Google Scholar] [CrossRef]
- Khusniyarov, M.M.; Weyhermueller, T.; Bill, E.; Wieghardt, K. Tuning the Oxidation Level, the Spin State, and the Degree of Electron Delocalization in Homo- and Heteroleptic Bis(α-Diimine)Iron Complexes. J. Am. Chem. Soc. 2009, 131, 1208–1221. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Yambulatov, D.S.; Cherkasov, A.V.; Demeshko, S.V. Europium Complexes with 1,2-Bis(Arylimino)Acenaphthenes: A Search for Redox Isomers. Russ. Chem. Bull. 2015, 64, 38–43. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Yambulatov, D.S.; Skatova, A.A.; Baranov, E.V.; Demeshko, S.; Bogomyakov, A.S.; Ovcharenko, V.I.; Zueva, E.M. Ytterbium and Europium Complexes of Redox-Active Ligands: Searching for Redox Isomerism. Inorg. Chem. 2017, 56, 9825–9833. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Magdesieva, T.V.; Levitskiy, O.A.; Korchagin, D.V.; Efimov, N.N.; Vasil’ev, P.N.; Goloveshkin, A.S.; Sidorov, A.A.; et al. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(Arylimino)Acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules 2020, 25, 2054. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Kholin, K.V.; Khrizanforov, M.N.; Budnikova, Y.G.; Babeshkin, K.A.; Efimov, N.N.; Goloveshkin, A.S.; Imshennik, V.K.; et al. Generation of a Hetero Spin Complex from Iron(II) Iodide with Redox Active Acenaphthene-1,2-Diimine. Molecules 2021, 26, 2998. [Google Scholar] [CrossRef] [PubMed]
- Romashev, N.F.; Abramov, P.A.; Bakaev, I.V.; Fomenko, I.S.; Samsonenko, D.G.; Novikov, A.S.; Tong, K.K.H.; Ahn, D.; Dorovatovskii, P.V.; Zubavichus, Y.V.; et al. Heteroleptic Pd(II) and Pt(II) Complexes with Redox-Active Ligands: Synthesis, Structure, and Multimodal Anticancer Mechanism. Inorg. Chem. 2022, 61, 2105–2118. [Google Scholar] [CrossRef]
- Biancalana, L.; Batchelor, L.K.; Dyson, P.J.; Zacchini, S.; Schoch, S.; Pampaloni, G.; Marchetti, F. α-Diimine Homologues of Cisplatin: Synthesis, Speciation in DMSO/Water and Cytotoxicity. New J. Chem. 2018, 42, 17453–17463. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Babeshkin, K.A.; Efimov, N.N.; Kiskin, M.A.; Eremenko, I.L. Synthesis, Structure, and Magnetic Properties of the Cobalt(Ii) Iodide Complex with 1,4-Diazabuta-1,3-Diene Ligand. Russ. Chem. Bull. 2021, 70, 2390–2396. [Google Scholar] [CrossRef]
- Beloglazkina, E.K.; Yudin, I.V.; Majouga, A.G.; Moiseeva, A.A.; Tursina, A.I.; Zyk, N. V Synthesis and Electrochemical Study of 2-(2-Pyridyl)Benzothiazole Complexes with Transition Metals (CoII, NiII, and CuII). Molecular Structure of Aquabis[2-(2-Pyridyl)Benzothiazole]Copper(II) Diperchlorate. Russ. Chem. Bull. 2006, 55, 1803–1809. [Google Scholar] [CrossRef]
- Comerlato, N.M.; Crossetti, G.L.; Howie, R.A.; Tibultino, P.C.D.; Wardell, J.L. Dichloro[N,N’-Bis(2,6-Diisopropylphenyl)-1,2-Ethanediimine-N.,N’]Palladium. Acta Crystallogr. Sect. E 2001, 57, m295–m297. [Google Scholar] [CrossRef] [Green Version]
- Rochon, F.D.; Melanson, R.; Howard-Lock, H.E.; Lock, C.J.L.; Turner, G. The Vibrational Spectra, Crystal and Molecular Structure of Bis(Acetonitrile)Dichloroplatinum(II). Can. J. Chem. 1984, 62, 860–869. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Odintsova, O.V.; Mikhaylov, V.N.; Sorokoumov, V.N.; Serebryanskaya, T.V.; Starova, G.L. Supramolecular Polymers Derived from the PtII and PdII Schiff Base Complexes via C(Sp2)–H … Hal Hydrogen Bonding: Combined Experimental and Theoretical Study. J. Organomet. Chem. 2019, 886, 71–75. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Druzhkov, N.O.; Fukin, G.K. Heterometallic Antimony(V)-Zinc and Antimony(V)-Copper Complexes Comprising Catecholate and Diazadiene as Redox Active Centers. J. Organomet. Chem. 2021, 952, 121994. [Google Scholar] [CrossRef]
- Ershova, I.V.; Meshcheryakova, I.N.; Trofimova, O.Y.; Pashanova, K.I.; Arsenyeva, K.V.; Khamaletdinova, N.M.; Smolyaninov, I.V.; Arsenyev, M.V.; Cherkasov, A.V.; Piskunov, A.V. Complexes of Metal Halides with Unreduced O-(Imino)Quinones. Inorg. Chem. 2021, 60, 12309–12322. [Google Scholar] [CrossRef]
- Cole, B.E.; Wolbach, J.P.; Dougherty, W.G.J.; Piro, N.A.; Kassel, W.S.; Graves, C.R. Synthesis and Characterization of Aluminum-α-Diimine Complexes over Multiple Redox States. Inorg. Chem. 2014, 53, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Bantreil, X.; Nolan, S.P. Synthesis of N-Heterocyclic Carbene Ligands and Derived Ruthenium Olefin Metathesis Catalysts. Nat. Protoc. 2011, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.A.; Labinger, J.A.; Bercaw, J.E. C−H Bond Activation by Cationic Platinum(II) Complexes: Ligand Electronic and Steric Effects. J. Am. Chem. Soc. 2002, 124, 1378–1399. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]






| Parameter | Value | |
|---|---|---|
| I | II | |
| Molecular formula | C26H36Cl2N2Pt | C22H28Cl2N2Pt |
| Mw, g mol−1 | 642.56 | 586.45 |
| T, K | 293 | 100 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | Cc |
| a, Å | 13.9314(13) | 22.738(3) |
| b, Å | 15.2288(14) | 7.2591(8) |
| c, Å | 14.0105(15) | 13.5245(15) |
| V, Å3 | 2759.6(5) | 2216.1(4) |
| β, deg | 111.816(4) | 96.903(4) |
| Z | 4 | 4 |
| ρcalcd, g cm−3 | 1.547 | 1.758 |
| μ, mm−1 | 5.293 | 6.582 |
| θ range, deg | 2.06–30.72 | 2.79–30.59 |
| F(000) | 1272 | 1144 |
| Index range | –20 ≤ h ≤ 19; –21 ≤ k ≤ 21; –19 ≤ l ≤ 19 | –31 ≤ h ≤ 31; –10 ≤ k ≤ 9; –18 ≤ l ≤ 18 |
| Number of reflections collected | 28,952 | 20,002 |
| Number of unique reflections | 8375 | 5791 |
| Rint | 0.0925 | 0.0616 |
| Number of reflections with I > 2σ(I) | 5911 | 5464 |
| GooF | 1.035 | 1.125 |
| R factor on F2 > 2σ(F2) | R1 = 0.0598, wR2 = 0.1483 | R1 = 0.0568, wR2 = 0.1486 |
| R factor (all data) | R1 = 0.0937, wR2 = 0.1633 | R1 = 0.0607, wR2 = 0.1527 |
| Δρmax/Δρmin, e/Å3 | 2.639/−2.586 | 10.197/−9.101 |
| I | II | |
|---|---|---|
| Pt-N | 2.000(6), 2.005(6) | 2.010(12), 2.015(12) |
| Pt-Cl | 2.2963(18), 2.2892(18) | 2.281(4), 2.301(4) |
| N-Cimine | 1.292(9), 1.304(9) | 1.30(2), 1.300(17) |
| N-CAr | 1.443(9), 1.446(9) | 1.434(17), 1.455(19) |
| Cimine-Cimine | 1.425(10) | 1.44(2) |
| Pt-N-Cimine | 114.9(5), 116.1(5) | 114.0(10), 115.0(10) |
| Pt-N-CAr | 124.3(4), 127.3(5) | 124.3(9), 124.6(9) |
| N-Pt-N | 78.5(2) | 79.3(5) |
| N-Pt-Cl | 96.15(17), 94.79(17) | 94.0(4), 96.3(3) |
| Cl-Pt-Cl | 90.62(7) | 90.68(15) |
| N-Cimine-Cimine-N | 0.1(10) | 5(2) |
| Cimine-N-CAr-Corto | 80.3(9), 95.7(9) | 90(2), 96.8(19) |
| Compound | Ered11/2, V | Ia/Ic | Ered2p, V |
|---|---|---|---|
| I | –0.40 | 0.92 | –1.75 |
| II | –0.75 | 0.82 | –1.78 |
| III | –0.71 | 0.92 | –1.84 |
| Cell Type | IC50 (μM) | |||
|---|---|---|---|---|
| I | II | III | CP | |
| SKOV3 | 95 | >100 | 12 | 6.5 |
| HDF | 49 | >100 | 9 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yambulatov, D.S.; Lutsenko, I.A.; Nikolaevskii, S.A.; Petrov, P.A.; Smolyaninov, I.V.; Malyants, I.K.; Shender, V.O.; Kiskin, M.A.; Sidorov, A.A.; Berberova, N.T.; et al. α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules 2022, 27, 8565. https://doi.org/10.3390/molecules27238565
Yambulatov DS, Lutsenko IA, Nikolaevskii SA, Petrov PA, Smolyaninov IV, Malyants IK, Shender VO, Kiskin MA, Sidorov AA, Berberova NT, et al. α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules. 2022; 27(23):8565. https://doi.org/10.3390/molecules27238565
Chicago/Turabian StyleYambulatov, Dmitriy S., Irina A. Lutsenko, Stanislav A. Nikolaevskii, Pavel A. Petrov, Ivan V. Smolyaninov, Irina K. Malyants, Victoria O. Shender, Mikhail A. Kiskin, Alexey A. Sidorov, Nadezhda T. Berberova, and et al. 2022. "α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity" Molecules 27, no. 23: 8565. https://doi.org/10.3390/molecules27238565