Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review
Abstract
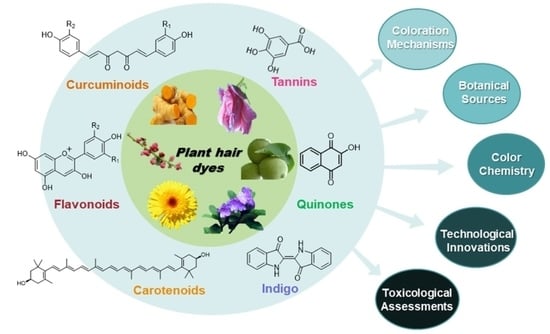
:1. Introduction
2. Hair Coloring Mechanisms

3. Phytochemicals Used for Hair Dyeing
3.1. Quinones
3.2. Tannins
3.3. Flavonoids
3.4. Indigo
3.5. Curcuminoids
3.6. Carotenoids
4. Technological Innovations for Natural Hair Dyeing
4.1. Colorant Production by Synthetic Biology Techniques
4.2. Encapsulation of Colorants for Stabilization and Detoxification
4.3. Development of Inorganic Nanocarriers for Efficient Hair Dyeing
5. Toxicological Assessments
6. Concluding Remarks
- Cumbersome extraction/purification procedures.
- High susceptibility to environmental pH, metal ions, UV and temperature.
- Low dye uptake and poor color fastness on hairs, especially unbleached hairs.
- Poor color reproducibility on human hair and testing models (e.g., yak hair and wool).
- Dependence on transition metallic mordant makes the dyed hair vulnerable to photo-oxidative damage and complicates the dyeing process.
- Insufficient toxicological data.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robbins, C.R. Dyeing Human Hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7, pp. 445–488. [Google Scholar]
- Ros, M.M.; Gago-Dominguez, M.; Aben, K.K.H.; Bueno-De-Mesquita, H.B.; Kampman, E.; Vermeulen, S.H.; Kiemeney, L.A. Personal hair dye use and the risk of bladder cancer: A case–control study from The Netherlands. Cancer Causes Control. 2012, 23, 1139–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morel, O.J.X.; Christie, R.M. Current Trends in the Chemistry of Permanent Hair Dyeing. Chem. Rev. 2011, 111, 2537–2561. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Yazar, K.; Hamann, C.R.; Thyssen, J.P.; Lidén, C. p-Phenylenediamine and other allergens in hair dye products in the United States: A consumer exposure study. Contact Dermat. 2014, 70, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Schuttelaar, M.-L.A.; Vogel, T.A. Contact Allergy to Hair Dyes. Cosmetics 2016, 3, 21. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Fautz, R.; Benech-Kieffer, F.; Toutain, H. Toxicity and human health risk of hair dyes. Food Chem. Toxicol. 2004, 42, 517–543. [Google Scholar] [CrossRef]
- Burnett, C.M.; Loehr, R.F.; Corbett, J.F. Dominant lethal mutagenicity study on hair dyes. J. Toxicol. Environ. Health Part A 1977, 2, 657–662. [Google Scholar] [CrossRef]
- Hp, C.; Reena, K.; Ng, K.Y.; Koh, R.Y.; Ch, N.; Chye, S.M. para-Phenylenediamine Containing Hair Dye: An Overview of Mutagenicity, Carcinogenicity and Toxicity. J. Environ. Anal. Toxicol. 2016, 6, 1000403. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Ali, A.; Moinuddin; Allarakha, S.; Fatima, S.; Ali, S.A.; Habib, S. Risk of Carcinogenicity Associated with Synthetic Hair Dyeing Formulations: A Biochemical View on Action Mechanisms, Genetic Variation and Prevention. Indian J. Clin. Biochem. 2022, 39, 399–409. [Google Scholar] [CrossRef]
- Boga, C.; Delpivo, C.; Ballarin, B.; Morigi, M.; Galli, S.; Micheletti, G.; Tozzi, S. Investigation on the dyeing power of some organic natural compounds for a green approach to hair dyeing. Dyes Pigment. 2013, 97, 9–18. [Google Scholar] [CrossRef]
- Beiki, T.; Najafpour, G.D.; Hosseini, M. Evaluation of antimicrobial and dyeing properties of walnut (Juglans regia L.) green husk extract for cosmetics. Color. Technol. 2017, 134, 71–81. [Google Scholar] [CrossRef]
- Dweck, A.C. Natural ingredients for colouring and styling. Int. J. Cosmet. Sci. 2002, 24, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Da França, S.A.; Dario, M.F.; Esteves, V.B.; Baby, A.R.; Velasco, M.V.R. Types of Hair Dye and Their Mechanisms of Action. Cosmetics 2015, 2, 110–126. [Google Scholar] [CrossRef] [Green Version]
- Oumeish, O.Y. The cultural and philosophical concepts of cosmetics in beauty and art through the medical history of mankind. Clin. Dermatol. 2001, 19, 375–386. [Google Scholar] [CrossRef]
- Tang, Y.; He, W.; Wu, Y.; Cai, R. Assessing the dyeing efficiency and irritation potentials of plant hair dyes: A multi-analytical in vitro approach. J. Cosmet. Dermatol. 2019, 18, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, W.; Yang, S.; Liu, L. Stabilisation and detoxification of henna (Lawsonia inermis L.) extract for hair dye cosmetics by spray-drying encapsulation. Color. Technol. 2019, 135, 439–450. [Google Scholar] [CrossRef]
- Himangshu, K.; Jyochhana Priya, M.; Gopal, P. Formulation and Evaluation of Natural Herbal Hair Dye Gel Using Lawsonia inermis (Henna Leaves) and Skin Irritation Studies in Albino Rats. J. Pharm. Pharmacol. 2022, 10, 18–24. [Google Scholar] [CrossRef]
- Boonsong, P.; Laohakunjit, N.; Kerdchoechuen, O. Natural pigments from six species of Thai plants extracted by water for hair dyeing product application. J. Clean. Prod. 2012, 37, 93–106. [Google Scholar] [CrossRef]
- Ali, S.; Maqbool, M.; Hussain, M.T. Efficacy of Some Plants Extracts for Natural Dyeing of Human Hair. J. Nat. Fibers 2020, 19, 2581–2595. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C. Formulation Studies and Properties Evaluation of Natural Semi-permanent Hair Dye Made from Gromwell Root and Sappan Wood. Sen’i Gakkaishi 2009, 65, 276–281. [Google Scholar] [CrossRef]
- Sivakumar, V.; Vijaeeswarri, J.; Anna, J.L. Effective natural dye extraction from different plant materials using ultrasound. Ind. Crops Prod. 2011, 33, 116–122. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols–A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Schäfer, N.; Balwierz, R.; Krzeszewska-Zaręba, A.; Skotnicki, Z.; Skotnicka-Graca, U.; Kalarus, K. Library of the University of Opole The use of botanical raw materials in hair dyeing. Aesthetic Cosmetol. Med. 2021, 10, 263–269. [Google Scholar] [CrossRef]
- Wei, G.; Bhushan, B.; Torgerson, P.M. Nanomechanical characterization of human hair using nanoindentation and SEM. Ultramicroscopy 2005, 105, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Swift, J.A.; Chahal, S.P.; Challoner, N.I.; Parfrey, J.E. Investigations on the penetration of hydrolyzed wheat proteins into human hair by confocal laser-scanning fluorescence microscopy. J. Cosmet. Sci. 2000, 51, 193–203. [Google Scholar]
- Kelch, A.; Wessel, S.; Will, T.; Hintze, U.; Wepf, R.; Wiesendanger, R. Penetration pathways of fluorescent dyes in human hair fibres investigated by scanning near-field optical microscopy. J. Microsc. 2000, 200, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Malinauskyte, E.; Shrestha, R.; Cornwell, P.A.; Gourion-Arsiquaud, S.; Hindley, M. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int. J. Cosmet. Sci. 2020, 43, 26–37. [Google Scholar] [CrossRef]
- Morel, O.; Christie, R.M.; Greaves, A.; Morgan, K.M. Enhanced model for the diffusivity of a dye molecule into human hair fibre based on molecular modelling techniques. Color. Technol. 2008, 124, 301–309. [Google Scholar] [CrossRef]
- Robbins, C.R.; Reich, C.; Patel, A. Adsorption to keratin surfaces: A continuum between a charge-driven and a hydrophobically driven process. J. Soc. Cosmet. Chem. 1994, 45, 85–94. [Google Scholar]
- Komboonchoo, S.; Bechtold, T. A study on the dyeing characteristics and electrochemical behaviour of lawsone–indigo mixtures. Color. Technol. 2011, 127, 153–158. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Continenza, M.A.; Vincenti, E.; Antonouli, S.; Torge, D.; Macchiarelli, G. Scanning Electron Microscopy Approach for Evaluation of Hair Dyed with Lawsonia inermis Powder: In vitro Study. Int. J. Morphol. 2020, 38, 96–100. [Google Scholar] [CrossRef]
- Rippon, D.M.L.J.A. The Coloration of Human Hair. The Coloration of Wool and Other Keratin Fibres; John Wiley & Sons: Bradford, UK, 2013; pp. 358–389. [Google Scholar]
- Mathur, J.P.; Gupta, N.P. Use of natural mordant in dyeing of wool. Indian J. Fibre Text. Res. 2003, 28, 90–93. [Google Scholar]
- Sargsyan, L.; Hippe, T.; Manneck, H.; Vill, V. Tannin-Mordant Coloration with Matcha (camelia sinensis) and Iron(II)-Lactate on Human Hair Tresses. Molecules 2021, 26, 829. [Google Scholar] [CrossRef] [PubMed]
- Mal, Ö.E.; Yıldırım, L. Metal mordants and biomordants. Impact Prospect. Green Chem. Text. Technol. 2019, 3, 57–82. [Google Scholar]
- Tang, Y.; Dyer, J.M.; Deb-Choudhury, S.; Li, Q. Trace metal ions in hair from frequent hair dyers in China and the associated effects on photo-oxidative damage. J. Photochem. Photobiol. B Biol. 2016, 156, 35–40. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Gharanjig, K.; Razani, N.; Jafari, R.; Saeb, M.R. Green miles in dyeing technology: Metal-rich pumpkin extracts in aid of natural dyes. Environ. Sci. Pollut. Res. 2022, 29, 50608–50616. [Google Scholar] [CrossRef]
- Packianathan, N.; Karumbayaram, S. Formulation and Evaluation of Herbal Hair Dye: An Ecofriendly Process. J. Pharm. Sci. Res. 2010, 2, 648–656. [Google Scholar]
- Rani, N.U.; Jajpura, L.; Butola, B.S. Ecological Dyeing of Protein Fabrics with Carica papaya L. Leaf Natural Extract in the Presence of Bio-mordants as an Alternative Copartner to Metal Mordants. J. Inst. Eng. India Ser. E 2020, 101, 19–31. [Google Scholar] [CrossRef]
- Gonzalez, V.; Wood, R.J.; Lee, J.; Taylor, S.; Bussemaker, M.J. Ultrasound-enhanced hair dye application for natural dyeing formulations. Ultrason. Sonochem. 2018, 52, 294–304. [Google Scholar] [CrossRef]
- Jin, S.; Sato, N. Benzoquinone, the substance essential for antibacterial activity in aqueous extracts from succulent young shoots of the pear Pyrus spp. Phytochemistry 2003, 62, 101–107. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, S.; He, W.; Liu, L.; Zhang, Z. Stabilization of Chinese Gallnut (Galla Chinensis) Tannins by Spray-Drying Microencapsulation for Natural Hair Coloring. Fibers Polym. 2020, 21, 1283–1292. [Google Scholar] [CrossRef]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of Anthocyanins from Blackcurrant ( Ribes nigrum L.) Fruit Waste as Renewable Hair Dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Inman, C.; Lourith, N.; Kanlayavattanakul, M. Alternative application approach on black bean: Hair coloring product. Chem. Biol. Technol. Agric. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Pipattanamomgkol, P.; Lourith, N.; Kanlayavattanakul, M. The natural approach to hair dyeing product with Cleistocalyx nervosum var. paniala. Sustain. Chem. Pharm. 2018, 8, 88–93. [Google Scholar] [CrossRef]
- Priprem, A.; Lee, Y.-C.; Limphirat, W.; Tiyaworanant, S.; Saodaeng, K.; Chotitumnavee, J.; Kowtragoon, N. Eucalyptus ash alters secondary protein conformation of human grey hair and facilitates anthocyanin dyeing. PLoS ONE 2018, 13, e0199696. [Google Scholar] [CrossRef]
- Tibkawin, N.; Suphrom, N.; Nuengchamnong, N.; Khorana, N.; Charoensit, P. Utilisation of Tectona grandis (teak) leaf extracts as natural hair dyes. Color. Technol. 2021, 138, 355–367. [Google Scholar] [CrossRef]
- Rao, Y.M.; Shayeda; Sujatha, P.S. Formulation and evaluation of commonly used natural hair colorants. Nat. Prod. Radiance 2008, 7, 45–48. [Google Scholar]
- Komboonchoo, S.; Bechtold, T. Sorption Characteristics of Indigo Carmine as a Blue Colorant for Use in One-bath Natural Dyeing. Text. Res. J. 2009, 80, 734–743. [Google Scholar] [CrossRef]
- Kadu, S.S. Phytochemical and Physicochemical screening of Curcuma longa Linn. Rhizome by using different solvents. GP Glob. Res. J. Chem. 2020, 3, 55–59. [Google Scholar]
- Dulo, B.; Phan, K.; Githaiga, J.; Raes, K.; De Meester, S. Natural Quinone Dyes: A Review on Structure, Extraction Techniques, Analysis and Application Potential. Waste Biomass-Valorization 2021, 12, 6339–6374. [Google Scholar] [CrossRef]
- Qiu, H.-Y.; Wang, P.; Lin, H.-Y.; Tang, C.-Y.; Zhu, H.-L.; Yang, Y.-H. Naphthoquinones: A continuing source for discovery of therapeutic antineoplastic agents. Chem. Biol. Drug Des. 2017, 91, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crops Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Tukenmez Demirci, G.; Kıvanç Altunay, İ.; Atış, G.; Kucukunal, A. Allergic contact dermatitis mimicking angioedema due to paraphenylendiamine hypersensitivity: A case report. Cutan. Ocul. Toxicol. 2012, 31, 250–252. [Google Scholar] [CrossRef]
- de Ávila, R.I.; Veloso, D.F.M.C.; Teixeira, G.C.; Rodrigues, T.L.; Lindberg, T.; Lindstedt, M.; Fonseca, S.G.; Lima, E.M.; Valadares, M.C. Evaluation of in vitro testing strategies for hazard assessment of the skin sensitization potential of “real-life” mixtures: The case of henna-based hair-colouring products containing p-phenylenediamine. Contact Dermat. 2019, 81, 194–209. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety (SCCS) Opinion on Lawsonia inermis (Henna) COLIPA n C169-SCCS/1511/13 Corrigendum, 12 November 2021. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_140.pdf (accessed on 14 November 2022).
- Syeda, N.F.; Hemalatha, G.; Smitha, T.; Rama, M. Assessment of Neuropharmacological Profile of Ethanolic Extract of Lawsonia Inermis Flowers. Mapana-J. Sci. 2020, 19, 37–48. [Google Scholar] [CrossRef]
- Mikhaeil, B.R.; Badria, F.A.; Maatooq, G.T.; Amer, M.M.A. Antioxidant and Immunomodulatory Constituents of Henna Leaves. Z. Für Nat. C 2004, 59, 468–476. [Google Scholar] [CrossRef]
- Nayak, B.S.; Isitor, G.; Davis, E.M.; Pillai, G.K. The evidence based wound healing activity of Lawsonia inermis Linn. Phytother. Res. 2007, 21, 827–831. [Google Scholar] [CrossRef]
- Vakilian, S.; Norouzi, M.; Soufi-Zomorrod, M.; Shabani, I.; Hosseinzadeh, S.; Soleimani, M. L. inermis-loaded nanofibrous scaffolds for wound dressing applications. Tissue Cell 2018, 51, 32–38. [Google Scholar] [CrossRef]
- Ramezani, N.; Raji, F.; Rezakazemi, M.; Younas, M. Juglone extraction from walnut (Juglans regia L.) green husk by supercritical CO2: Process optimization using Taguchi method. J. Environ. Chem. Eng. 2020, 8, 103776. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Sun, X.; Bai, B.; Jiang, D.; Wu, Z. Label-free quantitative proteomic analysis of the inhibitory activities of juglone against translation and energy metabolism in Escherichia coli. Phytochem. Lett. 2016, 18, 55–58. [Google Scholar] [CrossRef]
- Paulsen, M.T.; Ljungman, M. The natural toxin juglone causes degradation of p53 and induces rapid H2AX phosphorylation and cell death in human fibroblasts. Toxicol. Appl. Pharmacol. 2005, 209, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic Action of Juglone and Plumbagin: A Mechanistic Study Using HaCaT Keratinocytes. Chem. Res. Toxicol. 2003, 17, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Valipour, M. Recent advances of antitumor shikonin/alkannin derivatives: A comprehensive overview focusing on structural classification, synthetic approaches, and mechanisms of action. Eur. J. Med. Chem. 2022, 235, 114314. [Google Scholar] [CrossRef] [PubMed]
- Boulos, J.C.; Rahama, M.; Hegazy, M.-E.F.; Efferth, T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019, 459, 248–267. [Google Scholar] [CrossRef]
- Dhandapani, R.; Sarkar, A.K. Antibacterial activity and UV property of shikonin on silk substrate. J. Text. Appar. Technol. Manag. 2007, 5, 1–7. [Google Scholar]
- Malik, E.M.; Müller, C.E. Anthraquinones As Pharmacological Tools and Drugs. Med. Res. Rev. 2016, 36, 705–748. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and its Antiproliferative Activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Smart, K.; Abraham, M.H. Abraham model solute descriptors reveal strong intramolecular hydrogen bonding in 1,4-dihydroxyanthraquinone and 1,8-dihydroxyanthraquinone. Phys. Chem. Liq. 2017, 56, 416–420. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Hu, X.-D.; Zhu, J.-J.; Wan, J.; Yao, J.-B. Studies on the photofading of alizarin, the main component of madder. Dye. Dyes Pigment. 2021, 185, 108940. [Google Scholar] [CrossRef]
- Fain, V.Y.; Zaitsev, B.E.; Ryabov, M.A. Quantum-chemical and correlation study of ionization of Alizarin. Russ. J. Gen. Chem. 2004, 74, 1558–1563. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, N.; Faruk, O.; Ashaduzzaman; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Han, S.Y.; Hong, S.-P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron Gall Ink Revisited: Natural Formulation for Black Hair-Dyeing. Cosmetics 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Sargsyan, L.; Vill, V.; Hippe, T. Investigations of vegetable tannins as hair dyes and their interactions with pre-bleached hair fibres. Int. J. Cosmet. Sci. 2020, 42, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Burkinshaw, S.M.; Kumar, N. The mordant dyeing of wool using tannic acid and FeSO4, Part 1: Initial findings. Dyes Pigment. 2009, 80, 53–60. [Google Scholar] [CrossRef]
- Jahangiri, A.; Ghoreishian, S.M.; Akbari, A.; Norouzi, M.; Ghasemi, M.; Ghoreishian, M.; Shafiabadi, E. Natural Dyeing of Wool by Madder (Rubia tinctorum L.) Root Extract Using Tannin-based Biomordants: Colorimetric, Fastness and Tensile Assay. Fibers Polym. 2018, 19, 2139–2148. [Google Scholar] [CrossRef]
- Yusuf, M.; Shahid, M.; Khan, M.I.; Khan, S.; Mohammad, F. Dyeing studies with henna and madder: A research on effect of tin (II) chloride mordant. J. Saudi Chem. Soc. 2011, 19, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Adeel, S.; Kiran, S.; Yousaf, M.S. Eco-friendly isolation of tannin based natural colorant from coconut coir (Cocos nucifera) for dyeing of bio-mordanted wool fabric. Glob. NEST J. 2020, 23, 65–72. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E. Anthocyanin Pigments-Bioactivity and Coloring Properties. J. Food Sci. 2006, 69, C419–C425. [Google Scholar] [CrossRef]
- Houbiers, C.; Lima, J.C.; Maçanita, A.L.; Santos, H. Color Stabilization of Malvidin 3-Glucoside: Self-Aggregation of the Flavylium Cation and Copigmentation with the Z-Chalcone Form. J. Phys. Chem. B 1998, 102, 3578–3585. [Google Scholar] [CrossRef]
- Gao, L.; Gao, H. Haematoxylin sorption onto yak hair: Kinetic and thermodynamic studies. Color. Technol. 2013, 130, 21–26. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Shahiq-uz-zaman; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plants Res. 2012, 6, 1492–1496. [Google Scholar]
- Semwal, R.B.; Semwal, D.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin—A simple flavone exhibiting diverse pharmacological activities. Phytochem. Lett. 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Levic, S.; Kalusevic, A.; Špoljarić, I.; Đorđević, V.; Komes, D.; Mršić, G.; Nedovic, V. Efficiency Assessment of Natural Biopolymers as Encapsulants of Green Tea (Camellia sinensis L.) Bioactive Compounds by Spray Drying. Food Bioprocess. Technol. 2015, 8, 2444–2460. [Google Scholar] [CrossRef]
- Laleh, G.H.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare, S. The Effect of Light, Temperature, pH and Species on Stability of Anthocyanin Pigments in Four Berberis Species. Pak. J. Nutr. 2006, 5, 90–92. [Google Scholar]
- Yingjiao, Z.; Zhu, Y.; Jian, C.; Xia, C.; Deng, J.; Li, H.; Yanling, L.; Juan, L.; Pei, L. Identification of three species commonly known as “daqingye” by internal leaf anatomy and high-performance liquid chromatography analyses. Acta Soc. Bot. Pol. 2018, 87, 1–10. [Google Scholar]
- Qi-Yue, Y.; Ting, Z.; Ya-Nan, H.; Sheng-Jie, H.; Xuan, D.; Li, H.; Chun-Guang, X. From natural dye to herbal medicine: A systematic review of chemical constituents, pharmacological effects and clinical applications of indigo naturalis. Chin. Med. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xie, J.; Li, G.; Wang, F.; Lin, J.; Yang, M.; Du, A.; Zhang, D.; Han, L. Psoriasis treatment using Indigo Naturalis: Progress and strategy. J. Ethnopharmacol. 2022, 297, 115522. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, R.S.; Bechtold, T.; John, P. The development of indigo reduction methods and pre-reduced indigo products. Color. Technol. 2009, 125, 193–207. [Google Scholar] [CrossRef]
- Božič, M.; Kokol, V. Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigment. 2008, 76, 299–309. [Google Scholar] [CrossRef]
- Fabara, A.N.; Fraaije, M.W. An overview of microbial indigo-forming enzymes. Appl. Microbiol. Biotechnol. 2019, 104, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Qin, S. Pharmacokinetics Research on Anti-Inflammatory Effect and Analgesic Effect of Indigo Naturalis. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 137–140. [Google Scholar]
- Lin, Y.-K.; Leu, Y.-L.; Huang, T.-H.; Wu, Y.-H.; Chung, P.-J.; Pang, J.-H.S.; Hwang, T.-L. Anti-inflammatory effects of the extract of indigo naturalis in human neutrophils. J. Ethnopharmacol. 2009, 125, 51–58. [Google Scholar] [CrossRef]
- Chengaiah, B.; Rao, K.M.; Kumar, K.M.; Alagusundaram, M.; Chetty, C.M. Medicinal importance of natural dyes-a review. Int. J. PharmTech Res. 2010, 2, 144–154. [Google Scholar]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Bioprocess. 2022, 9, 1–38. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Zhou, J.; Li, S.; Liu, J.; Chen, H. Insight into the Progress on Natural Dyes: Sources, Structural Features, Health Effects, Challenges, and Potential. Molecules 2022, 27, 3291. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chemler, J.A.; Huang, L.L.; Martens, S.; Koffas, M.A.G. Metabolic Engineering of Anthocyanin Biosynthesis in Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 3617–3623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, J.L.; Couto, M.R.; Araújo, R.G.; Prather, K.L.; Kluskens, L.D.; Rodrigues, L.R. Hydroxycinnamic acids and curcumin production in engineered Escherichia coli using heat shock promoters. Biochem. Eng. J. 2017, 125, 41–49. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Wang, D.-N.; Chen, J.; Liu, Z.-J.; Wei, L.-J.; Hua, Q. Metabolic engineering of β-carotene biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Han, J.; Tang, Y.A. Encapsulating plant ingredients for dermocosmetic application: An updated review of delivery systems and characterization techniques. Int. J. Cosmet. Sci. 2019, 42, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application–a review. J. Microencapsul. 2015, 33, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Silva, M.; Martins, A.M.; Sousa-Oliveira, I.; Ribeiro, H.M.; Veiga, F.; Marto, J.; Paiva-Santos, A.C. Nanomaterials in hair care and treatment. Acta Biomater. 2022, 142, 14–35. [Google Scholar] [CrossRef]
- Rosen, J.; Landriscina, A.; Friedman, A.J. Nanotechnology-Based Cosmetics for Hair Care. Cosmetics 2015, 2, 211–224. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Z.; Yang, S.-Q.; Smith, G.J.; Liu, L. Diatomite encapsulated AgNPs as novel hair dye cosmetics: Preparation, performance, and toxicity. Colloids Surf. B. Biointerfaces 2021, 200, 111599. [Google Scholar] [CrossRef]
- Sampaio, S.; Maia, F.; Gomes, J.R. Diffusion of coloured silica nanoparticles into human hair. Color. Technol. 2010, 127, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Qiu, Y.; Gao, D.; Zhang, K.; Zhou, K.; Yin, H.; Yi, G.; Li, J.; Xia, Z.; Fu, Q. Melanin-mimetic multicolor and low-toxicity hair dye. RSC Adv. 2019, 9, 33617–33624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battistella, C.; McCallum, N.C.; Gnanasekaran, K.; Zhou, X.; Caponetti, V.; Montalti, M.; Gianneschi, N.C. Mimicking Natural Human Hair Pigmentation with Synthetic Melanin. ACS Central Sci. 2020, 6, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.; Fakhrullina, G.; Fakhrullin, R.F.; Lvov, Y.M. Self-assembly of clay nanotubes on hair surface for medical and cosmetic formulations. Nanoscale 2018, 10, 18205–18216. [Google Scholar] [CrossRef] [PubMed]
- Micó-Vicent, B.; Martínez-Verdú, F.M.; Novikov, A.A.; Stavitskaya, A.; Vinokurov, V.A.; Rozhina, E.; Fakhrullin, R.F.; Yendluri, R.; Lvov, Y.M. Stabilized Dye-Pigment Formulations with Platy and Tubular Nanoclays. Adv. Funct. Mater. 2018, 28, 1703553. [Google Scholar] [CrossRef] [Green Version]
- Groot, A.C. Side-effects of henna and semi-permanent 'black henna' tattoos: A full review. Contact Dermat. 2013, 69, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Metals in cosmetics: Implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Ababneh, F.A.; Al-Momani, I.F. Assessments of toxic heavy metals contamination in cosmetic products. Environ. Forensics 2018, 19, 134–142. [Google Scholar] [CrossRef]
- Pu, Z.-F.; Wu, B.-C.; Tan, Y.-H.; Wen, Q.-L.; Ling, J.; Cao, Q.-E. Selective Aggregation of Silver Nanoprisms Induced by Monohydrogen Phosphate and its Application for Colorimetric Detection of Chromium (III) Ions. J. Anal. Test. 2021, 5, 225–234. [Google Scholar] [CrossRef]
- Lai, Z.; Lin, F.; Huang, Y.; Wang, Y.; Chen, X. Automated Determination of Cd2+ and Pb2+ in Natural Waters with Sequential Injection Analysis Device Using Differential Pulse Anodic Stripping Voltammetry. J. Anal. Test. 2021, 5, 60–68. [Google Scholar] [CrossRef]
- Coimbra, S.C.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Peixoto, D.; Pereira-Silva, M.; Mathur, A.; Pawar, K.D.; Raza, F.; Mazzola, P.G.; Mascarenhas-Melo, F.; et al. Safety Assessment of Nanomaterials in Cosmetics: Focus on Dermal and Hair Dyes Products. Cosmetics 2022, 9, 83. [Google Scholar] [CrossRef]






| Category | Colorant | Botanical Origin | Extraction Process | Dye Bath | Dyeingsubstrate | Mordant | Dyeing Process | Dyed Color | Color Fastness | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|
| Quinones | lawsone | leaves of Lawonia inermis L. | ultrasound reflux extraction (sodium hydroxide solution 0.25 mol/L, solid liquid ratio 1:55, 140 min, 100 °C) | dye gels (xanthan gum, 1,2-propanediol) | gray hair | iron (II) sulfate | post-mordanting dyeing | reddish brown | / | [16] |
| lawsone | leaves of Lawonia inermis L. | reflux extraction (distilled water, solid liquid ratio 1:6, 120 min, 100 °C) | emulsion | gray hair | iron (II) sulfate | post-mordanting dyeing | blank | resistant to 20 shampoo washes | [20] | |
| lawsone | leaves of Lawonia inermis L. | ultrasound reflux extraction (sodium hydroxide 0.25 mol/L, solid liquid ratio 1:55, 140 min, 100 °C) | dye gels (xanthan gum, 1,2-propanediol) | yak hair | iron (II) sulfate | post-mordanting dyeing | reddish brown | resistant to 15 shampoo washes | [17] | |
| lawsone | leaves of Lawonia inermis L. | cold maceration extraction (water, 48 h) | dye gels (carbopol-934, glycerin, sodium hydroxide solution, methyl paraben) | hair | / | direct dyeing | brownish | resistant to 5 shampoo washes | [18] | |
| lawsone | leaves of Lawonia inermis L. | / | paste | goat hair | / | direct dyeing | reddish brown | / | [42] | |
| juglone | husk of Juglans regia L. | solvent extraction with microwave-assisted (acetone–water 70% (v/v), 60 s, 180 w) and ultrasound-assisted (20 min, 90 w, 37 kHz) | solution | bleached hair | iron (II) sulfate and aloe vera gel | meta-mordanting dyeing | dark brown | resistant to 15 shampoo washes | [12] | |
| juglone | husk of Juglans regia L. | ultrasound reflux extraction (ethanol 50%, solid liquid ratio 1:25, 120 min, 60 °C) | dye gels (xanthan gum, 1,2-propanediol) | gray hair | iron (II) sulfate | post-mordanting dyeing | brown | / | [16] | |
| juglone | husk of Juglans regia L. | Solvent extraction (dichloromethane, 60 min, 3 times) | solution | yak hair | / | direct dyeing | red brown | / | [11] | |
| shikonin | roots of Lithospermum erythrorhizon Sieb. et Zucc. | solvent extraction (ethanol and 3% acetic acid, 30 h) | solution | bleached hair | / | direct dyeing | light brown grey | resistant to 8 shampoo washes | [21] | |
| alizarin | roots of Rubia tinctoria L. | / | solution | yak hair | / | direct dyeing | red | / | [11] | |
| benzoquinone | shoots of Pyrus lindleyi Rehd. | solvent extraction (phosphate buffer, pH 6.0) | solution | yak hair | / | direct dyeing | brown | / | [11,43] | |
| Tannins | gallotannin | parasitic aphids of Rhus chinensis Mill. | ultrasound reflux extraction (80% ethanol, solid liquid ratio 1:25, 160 min, 60 °C) | dye gels (Xanthan gum, 1,2-propanediol) | gray hair | iron (II) sulfate | post-mordanting | black | resistant to 13 shampoo washes | [44] |
| gallotannin | parasitic aphids of Rhus chinensis Mill. | ultrasound reflux extraction (80% ethanol, solid liquid ratio 1:25, 160 min, 60 °C) | dye gels (Xanthan gum, 1,2-propanediol) | gray hair | iron (II) sulfate | post-mordanting | black | / | [44] | |
| catechin | matcha tea | / | solution | unpigmented hair | iron (II) lactate | post-mordanting | dove grey | resistant to 12 shampoo washes | [36] | |
| Flavonoids | cyanidin-3-o-rutinoside | fruit skins of Ribes nigrum L. | aqueous extraction (acidified water, 2 h) | paste | bleached hair | / | direct dyeing | blue | resistant to 12 shampoo washes | [45] |
| cyanidin-3-glucoside | fruit of Morus nigra L. | solvent extraction (methanol with aq. hydrochloric acid 1%, 30 min) | solution | yak hair | iron (II) oxalate | meta-mordanting | blue | / | [11] | |
| cyanidin-3-glucoside | beans of Phaseolus mungo | solvent extraction (hydrochloric ethanol, 4 °C, 24 h) | dye gels | bleached hair | / | direct dyeing | brownish red | resistant to 4 shampoo washes | [46] | |
| cyanidin-3-glucoside | fruit of Cleistocalyx nervosum Var. Paniala | solvent extraction (hydrochloric ethanol, 24 h) | spray | bleached hair | / | direct dyeing | red | resistant to 5 shampoo washes | [47] | |
| cyanidin-3-glucoside | corn cobs of Zea mays L. Var. | aqueous extraction (80 ± 2 °C, 15 min) | solution | grey hair | / | direct dyeing | blue | / | [48] | |
| hematoxylin | heartwood of Haematoxylon campechianum | ultrasound reflux extraction (ethanol 80%, solid liquid ratio1:25, 160 min, 60 °C) | dye gels (Xanthan gum, 1,2-propanediol) | gray hair | iron (II) sulfate | post-mordanting | brown red | / | [16] | |
| hematoxylin | heartwood of Haematoxylon campechianum | aqueous extraction (pH 9,25 °C, 1:4 (w/v)) | solution | bleached hair | iron (II) sulfate | meta-mordanting | reddish-brown | resistant to 15 shampoo washes | [19] | |
| hematoxylin | heartwood of Haematoxylon campechianum | aqueous extraction (95 °C, 50 min, 4 times) | solution | bleached hair | / | direct dyeing | light brown red | resistant to 8 shampoo washes | [21] | |
| quercetin | leaves of Tectona grandis Linn. F. | solvent extraction (ethanol-water, 70 °C, 3 h, 2 cycles) | solution | bleached hair | / | direct dyeing | brown | / | [49] | |
| acacetin | bark of Acacia farnesiana (Linn.) Willd. | reflux extraction with (distilled water, solid liquid ratio 1:6, 120 min, 100 °C) | solution | gray hair | direct dyeing | / | [20] | |||
| Indigo | indigo | leaves of Isatis indigotica Fort., Polygonum tinctorium Ait. | / | paste | gray hair | direct dyeing | dark brown | Resistant to 6 shampoo washes | [50] | |
| indigo carmine | / | / | solution | blonde hair | / | direct dyeing | blue | / | [51] | |
| Curcuminoids | curcumin | root of Curcuma longa Linn. | aqueous extraction (4 °C, pH 5, solid liquid ratio 1:4) | solution | bleached hair | iron (II) sulfate | meta-mordanting | orangish brown | resistant to 15 shampoo washes | [19] |
| curcumin | root of Curcuma longa Linn. | / | solution | yak hair | / | direct dyeing | yellow | / | [11,52] | |
| Carotenoids | zeaxanthin | aerial parts of Eclipta alba L. | aqueous extraction (100 °C, pH 9, solid liquid ratio 1:4) | solution | bleached hair | iron (II) sulfate | meta-mordanting | brown | resistant to 15 shampoo washes | [19] |
| peridinin | fruit of Terminalia belerica Roxb. | aqueous extraction (25 °C, pH 7, solid liquid ratio 1:4) | solution | bleached hair | iron (II) sulfate | meta-mordanting | brown | resistant to 15 shampoo washes | [19] | |
| lutein | flower of Tagetes erecta Linn. | aqueous extraction (60 min, 100 °C) | solution | grey hair | aloe vera gel | meta-mordanting | black | resistant to 5 shampoo washes | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Xie, W.; Hua, Z.; Cao, L.; Xiong, Z.; Tang, Y.; Yuan, Z. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules 2022, 27, 8062. https://doi.org/10.3390/molecules27228062
Cui H, Xie W, Hua Z, Cao L, Xiong Z, Tang Y, Yuan Z. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules. 2022; 27(22):8062. https://doi.org/10.3390/molecules27228062
Chicago/Turabian StyleCui, Hongyan, Wenjing Xie, Zhongjie Hua, Lihua Cao, Ziyi Xiong, Ying Tang, and Zhiqin Yuan. 2022. "Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review" Molecules 27, no. 22: 8062. https://doi.org/10.3390/molecules27228062