Phytochemical Compounds and Pharmacological Properties of Larrea tridentata
Abstract
:1. Introduction
2. Methodology
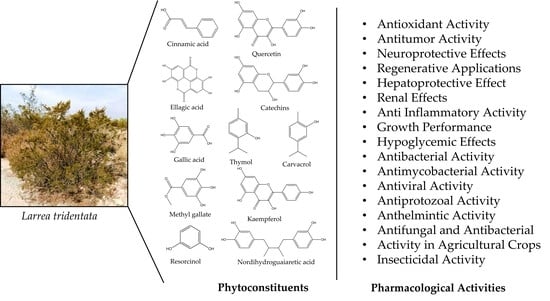
3. Phytoconstituents
Isolation of New Compounds
4. Pharmacological Activities
4.1. Antioxidant Activity
4.2. Antitumor Activity
4.3. Neuroprotective Effects
4.4. Regenerative Applications
4.5. Hepatoprotective Effect
4.6. Renal Effects
4.7. Anti-Inflammatory Activity
4.8. Growth Performance
4.9. Hypoglycemic Effects
4.10. Antibacterial Activity
4.11. Antimycobacterial Activity
4.12. Antiviral Activity
4.13. Antiprotozoal Activity
4.14. Anthelmintic Activity
4.15. Antifungal and Antibacterial Activity in Agricultural Crops
4.16. Insecticidal Activity
5. Side Effects
6. Discussion and Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodson, R.E.; Schery, R.W.; Porter, D.M. Flora of Panama: Part VI. Family 88. Zygophyllaceae. Ann. Mo. Bot. Gard. 1969, 56, 1–7. [Google Scholar] [CrossRef]
- Arteaga, S.; Andrade-Cetto, A.; Cárdenas, R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 2005, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Integrated Taxonomy Information System (ITIS). Larrea tridentata (DC.) Coville. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=29051#null (accessed on 21 August 2020).
- Rzedowski, J.; Calderón de Rzedowski, G. Zygophyllaceae. In Flora del Bajío y de Regions Adyacentes; Rzedowski, J., Calderón de Rzedowski, G., Eds.; Instituto de Ecología: Veracruz, México, 1994; Volume 30, pp. 11–15. [Google Scholar]
- Saldívar, R.H.L. Estado actual del conocimiento sobre las propiedades biocidas de la gobernadora [Larrea tridentata (DC) Coville]. Rev. Mex. Fitopatol. 2003, 21, 214–222. [Google Scholar]
- Gnabre, J.; Bates, R.; Huang, R.C. Creosote bush lignans for human disease treatment and prevention: Perspectives on combination therapy. J. Tradit. Complement. Med. 2015, 5, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Manda, G.; Rojo, A.I.; Martínez-Klimova, E.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic Acid: From Herbal Medicine to Clinical Development for Cancer and Chronic Diseases. Front. Pharmacol. 2020, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Zygophyllaceae. In Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions; Elsevier: Amsterdam, The Netherlands, 2015; pp. 611–612. [Google Scholar]
- Ruiz, J.; Ascacio-Valdés, J.; Rodriguez, R.; Morales, D.; Aguilar, C. Phytochemical screening of extracts from some Mexican plants used in traditional medicine. J. Med. Plants 2011, 5, 2791–2797. [Google Scholar]
- Martins, S.; Aguilar, C.N.; Teixeira, J.A.; Mussatto, S.I. Bioactive compounds (phytoestrogens) recovery from Larrea tridentata leaves by solvents extraction. Sep. Purif. Technol. 2012, 88, 163–167. [Google Scholar] [CrossRef]
- Ascacio-Valdés, J.A.; Aguilera-Carbó, A.; Rodríguez-Herrera, R.; Aguilar-González, C. Análisis de ácido elágico en algunas plantas del semidesierto mexicano. Rev. Mex. Cienc. Farm. 2013, 44, 36–40. [Google Scholar]
- Delgadillo-Ruíz, L.; Bañuelos-Valenzuela, R.; Delgadillo-Ruíz, O.; Silva-Vega, M.; Gallegos-Flores, P. Composición química y efecto antibacteriano in vitro de extractos de Larrea tridentata, origanum vulgare, artemisa ludoviciana y ruta graveolens. Nova Sci. 2017, 9, 273–290. [Google Scholar] [CrossRef]
- Bañuelos-Valenzuela, R.; Delgadillo-Ruiz, L.; Echavarría-Cháirez, F.; Delgadillo-Ruiz, O.; Meza-López, C. Composición química y FTIR de extractos etanólicos de Larrea tridentata, Origanum vulgare, Artemisa ludoviciana y Ruta graveolens. Agrociencia 2018, 52, 309–321. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5281855, Ellagic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ellagic-acid (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 370, Gallic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Gallic-acid (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1203, Epicatechin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Epicatechin (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7428, Methyl Gallate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methyl-gallate (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 444539, Cinnamic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cinnamic-acid (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5054, Resorcinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Resorcinol (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280863, Kaempferol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Kaempferol (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5280343, Quercetin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Quercetin (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4534, Nordihydroguaiaretic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Nordihydroguaiaretic-acid (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6989, Thymol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Thymol (accessed on 5 March 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 10364, Carvacrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carvacrol (accessed on 5 March 2021).
- Jitsuno, M.; Mimaki, Y. Triterpene glycosides from the aerial parts of Larrea tridentata. Phytochemistry 2010, 71, 2157–2167. [Google Scholar] [CrossRef]
- Yokosuka, A.; Matsuo, Y.; Jitsuno, M.; Adachi, K.; Mimaki, Y. Larrealignans A and B, Novel Lignan Glycosides from the Aerial Parts of Larrea tridentata. Chem. Pharm. Bull. 2011, 59, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Favela-Hernández, J.M.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. Phytother. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Rzeppa, S.; Kaiser, M.; Brun, R. Larrea tridentata—Absolute configuration of its epoxylignans and investigations on its antiprotozoal activity. Phytochem. Lett. 2012, 5, 632–638. [Google Scholar] [CrossRef]
- Núñez-Mojica, G.; Vázquez-Ramírez, A.L.; García, A.; Rivas-Galindo, V.M.; Garza-González, E.; Cuevas González-Bravo, G.E.; Toscano, R.A.; Moo-Puc, R.E.; Villanueva-Toledo, J.R.; Marchand, P.; et al. New cyclolignans of Larrea tridentata and their antibacterial and cytotoxic activities. Phytochem. Lett. 2021, 43, 212–218. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.; Aguilar, C.; Teixeira, J. Antioxidant capacity and NDGA content of Larrea tridentata (a desert bush) leaves extracted with different solvents. J. Biotechnol. 2010, 150, 500. [Google Scholar] [CrossRef]
- Rahman, S.; Ansari, R.A.; Rehman, H.; Parvez, S.; Raisuddin, S. Nordihydroguaiaretic Acid from Creosote Bush (Larrea tridentata) Mitigates 12-O-Tetradecanoylphorbol-13-Acetate-Induced Inflammatory and Oxidative Stress Responses of Tumor Promotion Cascade in Mouse Skin. Evid.-Based Complementary Altern. Med. 2011, 2011, 734–785. [Google Scholar] [CrossRef]
- Aguirre-Joya, J.A.; Pastrana-Castro, L.; Nieto-Oropeza, D.; Ventura-Sobrevilla, J.; Rojas-Molina, R.; Aguilar, C.N. The physicochemical, antifungal and antioxidant properties of a mixed polyphenol based bioactive film. Heliyon 2018, 4, e00942. [Google Scholar] [CrossRef]
- Skouta, R.; Morán-Santibañez, K.; Valenzuela, C.A.; Vasquez, A.H.; Fenelon, K. Assessing the Antioxidant Properties of Larrea tridentata Extract as a Potential Molecular Therapy against Oxidative Stress. Molecules 2018, 23, 1826. [Google Scholar] [CrossRef] [PubMed]
- Morán-Santibañez, K.; Vasquez, A.H.; Varela-Ramirez, A.; Henderson, V.; Sweeney, J.; Odero-Marah, V.; Fenelon, K.; Skouta, R. Larrea tridentata Extract Mitigates Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. Antioxidants 2019, 8, 427. [Google Scholar] [CrossRef]
- Vázquez-Cervantes, G.I.; Villaseñor-Aguayo, K.; Hernández-Damián, J.; Aparicio-Trejo, O.; Medina-Campos, O.N.; López-Marure, R.; Pedraza-Chaverri, J. Antitumor Effects of Nordihydroguaiaretic Acid (NDGA) in Bladder T24 Cancer Cells are Related to Increase in ROS Production and Mitochondrial Leak Respiration. Nat. Prod. Commun. 2018, 13, 1523–1526. [Google Scholar] [CrossRef]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Wu, J.; Xing, D.M.; Liu, Y.; Jia, F.H.; Li, D.T. Nordihydroguaiaretic Acid (NDGA) Promotes Functional Recovery after Transient Focal Cerebral Ischemia in Rats. Lat. Am. J. Pharm. 2014, 33, 994–1000. [Google Scholar]
- Kim, H.G.; Oh, M.S. Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 57–75. [Google Scholar] [PubMed]
- Siddique, Y.H.; Ali, F. Protective effect of nordihydroguaiaretic acid (NDGA) on the transgenic Drosophila model of Alzheimer’s disease. Chem.-Biol. Interact. 2017, 269, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Carrillo, K.L.; Saucedo-Acuña, R.A.; Ríos-Arana, J.; Tamayo, G.; Guzmán-Gastellum, D.A.; Díaz-Torres, B.A.; Nava-Martínez, S.D.; Espinosa-Cristóbal, L.F.; Cuevas-González, J.C. Synthesis, Characterization, and In Vitro and In Vivo Evaluations of Cellulose Hydrogels Enriched with Larrea tridentata for Regenerative Applications. BioMed. Res. Int. 2020, 2020, 1425402. [Google Scholar] [CrossRef]
- Del Vecchyo-Tenorio, G.; Rodríguez-Cruz, M.; Andrade-Cetto, A.; Cárdenas-Vázquez, R. Creosote Bush (Larrea tridentata) Improves Insulin Sensitivity and Reduces Plasma and Hepatic Lipids in Hamsters Fed a High Fat and Cholesterol Diet. Front. Pharmacol. 2016, 7, 194. [Google Scholar] [CrossRef]
- Chan, J.K.W.; Bittner, S.; Bittner, A.; Atwal, S.; Shen, W.J.; Inayathullah, M.; Rajada, J.; Nicolls, M.R.; Kraemer, F.B.; Azhar, S. Nordihydroguaiaretic Acid, a Lignan from Larrea tridentata (Creosote Bush), Protects against American Lifestyle-Induced Obesity Syndrome Diet-Induced Metabolic Dysfunction in Mice. J. Pharmacol. Exp. Ther. 2018, 365, 281–290. [Google Scholar] [CrossRef]
- Han, L.; Bittner, S.; Dong, D.; Cortez, Y.; Dulay, H.; Arshad, S.; Shen, W.J.; Kraemer, F.B.; Azhar, S. Creosote bush-derived NDGA attenuates molecular and pathological changes in a novel mouse model of non-alcoholic steatohepatitis (NASH). Mol. Cell Endocrinol. 2019, 498, 110538. [Google Scholar] [CrossRef]
- Zuntilde, A.; Tapia, E.; Zazueta, C.; Correa, F.; Zatarain-Barron, Z.L.; Hernandez, P.R.; Zarco-Marquez, G.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid pretreatment prevents ischemia and reperfusion induced renal injury, oxidant stress and mitochondrial alterations. J. Med. Plants Res. 2012, 6, 2938–2947. [Google Scholar]
- Zúñiga-Toalá, A.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Negrette-Guzmán, M.; Huerta-Yepez, S.; Torres, I.; Pinzón, E.; Tapia, E.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid induces Nrf2 nuclear translocation in vivo and attenuates renal damage and apoptosis in the ischemia and reperfusion model. Phytomedicine 2013, 20, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Medina-Campos, O.N.; Rada, P.; Zúñiga-Toalá, A.; López-Gazcón, A.; Espada, S.; Pedraza-Chaverri, J.; Cuadrado, A. Signaling pathways activated by the phytochemical nordihydroguaiaretic acid contribute to a Keap1-independent regulation of Nrf2 stability: Role of glycogen synthase kinase-3. Free Radic. Biol. Med. 2012, 52, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhang, X.Y.; Liu, J.M.; Song, Y.; Liu, T.T.; Chen, D. NDGA reduces secondary damage after spinal cord injury in rats via anti-inflammatory effects. Brain Res. 2013, 1516, 83–92. [Google Scholar] [CrossRef] [PubMed]
- García-López, J.C.; Lee-Rangel, H.A.; López, S.; Vicente, J.; Pardio-Sedas, V.; Estrada-Coates, A.T.; Pinos-Rodríguez, J.M. Effects of Larrea tridentata on growth, organ weights and hepatic enzymes of broilers. Agrociencia 2018, 52, 1–8. [Google Scholar]
- Hernández-Baez, I.; García-López, J.C.; Espinosa-Reyes, G.; Lee-Rangel, H.A.; Faz-Colunga, D.A.; Pinos-Rodríguez, J.M. Biomasa de gobernadora (Larrea tridentata) como forraje para borregos. Rev. Chapingo Serie Zonas Áridas 2019, 18, 1–9. [Google Scholar] [CrossRef]
- Roškar, I.; Štrukelj, B.; Lunder, M. Screening of Phenolic Compounds Reveals Inhibitory Activity of Nordihydroguaiaretic Acid against Three Enzymes Involved in the Regulation of Blood Glucose Level. Plant Foods Hum. Nutr. 2016, 71, 88–89. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.; Clemente-Soto, A.F.; Balderas-Rentería, I.; Garza-González, E.; del Camacho-Corona, M. Potential mechanism of action of 3’-demethoxy-6-O-demethyl-isoguaiacin on methicillin resistant Staphylococcus aureus. Molecules 2015, 20, 12450–12458. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.; Rodríguez, R.; Ruiz, J.; Morales-Adame, D.; Castillo, F.; Hernández-Castillo, F.D.; Aguilar, C.N. Antibacterial activity of plant extracts obtained with alternative organics solvents against food-borne pathogen bacteria. Ind. Crops Prod. 2012, 37, 445–450. [Google Scholar] [CrossRef]
- Snowden, R.; Harrington, H.; Morrill, K.; Jeane, L.; Garrity, J.; Orian, M.; Lopez, E.; Rezaie, S.; Hassberger, K.; Familoni, D.; et al. A comparison of the anti-Staphylococcus aureus activity of extracts from commonly used medicinal plants. J. Altern. Complement. Med. 2014, 20, 375–382. [Google Scholar] [CrossRef]
- Martins, S.; Amorim, E.L.C.; Sobrinho, T.J.S.P.; Saraiva, A.M.; Pisciottano, M.N.C.; Aguilar, C.N.; Teixeira, J.A.; Mussatto, S.I. Antibacterial activity of crude methanolic extract and fractions obtained from Larrea tridentata leaves. Ind. Crops Prod. 2013, 41, 306–311. [Google Scholar] [CrossRef]
- Cunningham-Oakes, E.; Soren, O.; Moussa, C.; Rathor, G.; Liu, Y.; Coates, A.; Hu, Y. Nordihydroguaiaretic acid enhances the activities of aminoglycosides against methicillin-sensitive and resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 2015, 6, 1195. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Melo, K.; García, A.; Romo-Mancillas, A.; Garza-González, E.; Rivas-Galindo, V.M.; Miranda, L.D.; Vargas-Villarreal, J.; Favela-Hernández, J.M.J.; del Camacho-Corona, M.R. meso-Dihydroguaiaretic acid derivatives with antibacterial and antimycobacterial activity. Bioorg. Med. Chem. 2017, 25, 5247–5259. [Google Scholar] [CrossRef] [PubMed]
- Gerstel, J.; Turner, T.; Ruiz, G.; Wise, J.; Stein, A.; Jones, G.; Morin, T.; Pinazza, T.; Sukhorukov, E.; Clark, D.; et al. Identification of botanicals with potential therapeutic use against methicillin-resistant Staphylococcus aureus (MRSA) infections. Phytother. Res. 2018, 32, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Itzá-Ortiz, M.; Chávez, J.; Urquizo, E.; Suescún, J. Phytobiotic activity of Larrea tridentata, Origanum vulgare and Plectranthus amboinicus in gram positive and gram negative bacterias. Interciencia 2019, 44, 298–302. [Google Scholar]
- Núñez-Mojica, G.; Hernández-Carrillo, M.L.; Avalos-Alanís, F.G.; Garza-González, E.; Rivas-Galindo, V.M.; Silva-Mares, D.A.; Camacho-Corona, M.R. Amino ether analogues of 4,4′-dihydroxy-3-methoxy-6,7′-cyclolignan and their activity against drug-resistant bacteria. Phytochem. Lett. 2022, 50, 57–60. [Google Scholar] [CrossRef]
- Turner, T.; Ruiz, G.; Gerstel, J.; Langland, J. Characterization of the antibacterial activity from ethanolic extracts of the botanical, Larrea tridentata. BMC Complement. Med. Ther. 2021, 21, 177. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Gonzalez-Cortazar, M.; Zaragoza-Bastida, A.; Meza-Nieto, M.A.; Valladares-Carranza, B.; Alsayegh, A.A.; El-Saber, B.G.; Rivero-Perez, N. nor 3’-Demethoxyisoguaiacin from Larrea tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis. Molecules 2022, 27, 3620. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Tuberculosis. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 17 September 2021).
- Clemente-Soto, A.F.; Balderas-Rentería, I.; Rivera, G.; Segura-Cabrera, A.; del Garza-González, E.R.; Camacho-Corona, M. Potential mechanism of action of meso-dihydroguaiaretic acid on Mycobacterium tuberculosis H37Rv. Molecules 2014, 19, 20170–20182. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, S.; Rubio-Badillo, M.Á.; Juárez, E.; Hernández-Sánchez, F.; Torres, M. Nordihydroguaiaretic acid (NDGA) and α-mangostin inhibit the growth of Mycobacterium tuberculosis by inducing autophagy. Int. Immunopharmacol. 2016, 31, 149–157. [Google Scholar] [CrossRef]
- Pollara, J.J.; Laster, S.M.; Petty, I.T.D. Inhibition of poxvirus growth by Terameprocol, a methylated derivative of nordihydroguaiaretic acid. Antivir. Res. 2010, 88, 287–295. [Google Scholar] [CrossRef]
- Camacho-Corona, M.R.; García., A.; Mata-Cárdenas, B.D.; Garza-González, E.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Bah, M.; Sánchez, M.A.Z.; Gutierrez, S.P. Screening for antibacterial and antiprotozoal activities of crude extracts derived from Mexican medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 104–112. [Google Scholar] [CrossRef]
- Bashyal, B.; Li, L.; Bains, T.; Debnath, A.; LaBarbera, D.V. Larrea tridentata: A novel source for anti-parasitic agents active against Entamoeba histolytica, Giardia lamblia and Naegleria fowleri. PLoS Negl. Trop. Dis. 2017, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- García, J.E.; Gómez, L.; Mendoza-de-Gives, P.; Rivera-Corona, J.L.; Millán-Orozco, J.; Ascacio, J.A.; Medina, M.A.; Mellado, M. Anthelmintic efficacy of hydro-methanolic extracts of Larrea tridentata against larvae of Haemonchus contortus. Trop Anim. Health Prod. 2018, 50, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.; Hernández, D.; Gallegos, G.; Mendez, M.; Rodríguez, R.; Reyes, A.; Aguilar, C.N. In vitro antifungal activity of plant extracts obtained with alternative organic solvents against Rhizoctonia solani Kühn. Ind. Crops Prod. 2010, 32, 324–328. [Google Scholar] [CrossRef]
- Osorio, E.; Flores, M.; Hernández, D.; Ventura, J.; Rodríguez, R.; Aguilar, C.N. Biological efficiency of polyphenolic extracts from pecan nuts shell (Carya Illinoensis), pomegranate husk (Punica granatum) and creosote bush leaves (Larrea tridentata Cov.) against plant pathogenic fungi. Ind. Crops Prod. 2010, 31, 153–157. [Google Scholar] [CrossRef]
- Chávez-Solís, A.L.; Pedroza-Sandoval, A.; Nava-Díaz, C.; Cano-Rios, P.; Castro-Franco, R. Control de la cenicilla del melón (Podosphaera xanthii) mediante el uso de extracto de Larrea tridentata (DC) Coville (I.). RChSZA 2014, 13, 103–113. [Google Scholar] [CrossRef]
- Galván, J.V.; Díaz, C.A.G.; Fernández, R.G. Efecto de los extractos acuosos de hojas de plantas de gobernadora (Larreas tridentata), hojas en (Flourensia cernua) y encino (Quercus pungens), sobre el crecimiento micelial in vitro de hongos fitopatógenos. Act. Univ. 2014, 24, 13–19. [Google Scholar]
- Munguía, A.R.; Zárate, M.A.; Inungaray, M.L.C. Efecto del uso combinado de extracto de Larrea tridentata y sorbato de potasio sobre el crecimiento de Aspergillus flavus. ReIbCi 2014, 1, 263–267. [Google Scholar]
- Castillo-Reyes, F.; Hernández-Castillo, F.D.; Clemente-Constantino, J.A.; Gallegos-Morales, G.; Rodríguez-Herrera, R.; Aguilar, C. In vitro antifungal activity of polyphenols-rich plant extracts against Phytophthora cinnamomi Rands. Afr. J. Agric. Res. 2015, 10, 4554–4560. [Google Scholar] [CrossRef]
- Peñuelas-Rubio, O.; Arellano-Gil, M.; Vargas-Arispuro, I.d.C.; Lares-Villa, F.; Cantú-Soto, E.; Hernández-Rodríguez, S.; Gutiérrez-Coronado, M.A.; Mungarro-Ibarra, C. Bioactividad in vitro de extractos de gobernadora (Larrea tridentata) sobre la inhibición de hongos poscosecha: Alternaria tenuissima, Aspergillus niger, Penicillium polonicum y Rhizopus oryzae. Polibotánica 2015, 40, 183–198. [Google Scholar] [CrossRef]
- Peñuelas-Rubio, O.; Arellano-Gil, M.; Verdugo-Fuentes, A.A.; Chaparro-Encinas, L.A.; Hernández-Rodríguez, S.E.; Martínez-Carrillo, J.L.; Vargas-Arispuro, I.d.C. Extractos de Larrea tridentata como una estrategia ecológica contra Fusarium oxysporum radicis-lycopersici en plantas de tomate bajo condiciones de invernadero. Rev. Mex. Fitopatol. 2017, 35, 360–376. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Avila-Ramos, F.; Aquino-Torres, E.; Prieto-Méndez, J.; Hetta, H.F.; El-Saber, B.G.; Zaragoza-Bastida, A. Bactericidal Activity of Larrea tridentata Hydroalcoholic Extract against Phytopathogenic Bacteria. Agronomy 2021, 11, 957. [Google Scholar] [CrossRef]
- Méndez-Andrade, R.; Vallejo-Perez, M.R.; Loera-Alvarado, E.; de los Santos-Villarreal, G.; García-Cerda, L.A.; Vera-Reyes, I. Efficacy of biosynthesized silver nanoparticles from Larrea tridentata against Clavibacter michiganensis. J. Phytopathol. 2022, 170, 91–99. [Google Scholar] [CrossRef]
- Marín-Domínguez, M.; Pérez-Leal, R.; Núñez-Barrios, A.; Basurto-Sotelo, M.; Soto-Parra, J.M. Exposition of Pecan Black Aphid (Melanocallis caryaefoliae) to creseote bush (Larrea tridentata) extracts. J. Agric. Sci. 2014, 5, 1369. [Google Scholar] [CrossRef]
- Maldonado-Simán, E.; Chavarría-Sánchez, P.A.; Martinez-Hernandez, P.A.; Amendola-Massiotti, R.D.; Gonzalez-Garduno, R.; Hernandez-Valencia, E. Horn fly (Haematobia irritans) incidence on cows sprayed with creosote-bush (Larrea tridentata (DC.) Coville) leaf extract. Agrociencia 2018, 52, 323–331. [Google Scholar]
- Galarza-Tristán, F.; Aldama-Aguilera, C.; Hipólito-Cruz, G.; González-Montero, R.; Medellín-Castillo, N.; Bernal-Jacomé, L. Extractos vegetales para el control de larvas de mosquitos en diferentes calidades de agua de la ciudad de SLP. Entomol. Mex. 2018, 5, 148–154. [Google Scholar]
- Larrey, D.; Faure, S. Herbal medicine hepatotoxicity: A new step with development of specific biomarkers. J. Hepatol. 2011, 54, 599–601. [Google Scholar] [CrossRef]
- Higuera-de la Tijera, M.d.F.; Servín-Caamaño, A.I.; Alexanderson-Rosas, E.G. Toxicidad hepática inducida por fármacos y herbolaria. Rev. Med. Hosp. Gen. 2012, 75, 230–237. [Google Scholar]
- Brown, A.C. Kidney toxicity related to herbs and dietary supplements: Online table of case reports. Part 3 of 5 series. Food Chem. Toxicol. 2017, 107, 502–519. [Google Scholar] [CrossRef]
- Kotsiou, A.; Christine, T. Hepatotoxicity of herbal medicinal products. J. Med. Plant Stud. 2017, 5, 80–88. [Google Scholar]
- Vilas-Boas, V.; Gijbels, E.; Jonckheer, J.; De Waele, E.; Vinken, M. Cholestatic liver injury induced by food additives, dietary supplements and parenteral nutrition. Environ. Int. 2020, 136, 105422. [Google Scholar] [CrossRef] [PubMed]
| Taxonomy | |
|---|---|
| Kingdom | Plantae |
| Division | Tracheophyta |
| Class | Magnoliopsida |
| Order | Zygophyllales |
| Family | Zygophyllaceae |
| Genus | Larrea |
| Species | Tridentata |
| Compound | Class of Compound | IUPAC Name | Chemical Structure |
|---|---|---|---|
| Ellagic acid | organic heterotetracyclic compound, polyphenol | 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo [6.6.2.04,16.011,15]hexadeca-1(15),4,6,8(16),11,13-hexaene-3,10-dione |  |
| Gallic acid | trihydroxybenzoic acid | 3,4,5-trihydroxybenzoic acid |  |
| Catechins | Hydroxyflavanoids | 2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol |  |
| Methyl gallate | Gallate ester | methyl 3,4,5-trihydroxybenzoate |  |
| Cinnamic acid | Monocarboxylic acid, a styrene | (E)-3-phenylprop-2-enoic acid |  |
| Resorcinol | Benzenediol | benzene-1,3-diol |  |
| Kaempferol | Flavonol (tetrahydroxyflavone) | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one |  |
| Quercetin | Flavonoid | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one |  |
| Nordihydroguaiaretic acid (NDGA) | Lignan | 4-[4-(3,4-dihydroxyphenyl)-2,3-dimethylbutyl]benzene-1,2-diol |  |
| Thymol | Monoterpene | 5-methyl-2-propan-2-ylphenol |  |
| Carvacrol | Monoterpene | 2-methyl-5-propan-2-ylphenol |  |
| Organ-Extract | Compound | Class of Compound |
|---|---|---|
| Aerial parts, methanolic extract | 3-[(O-(4-O-sulfo-b-d-glucopyranosyl)-(1→3)-a-L-arabinopyranosyl) oxy]olean-12-en-28-oic acid b-d-glucopyranosyl ester sodium salt 3-[(O-(4-O-sulfob-d-glucopyranosyl)-(1→3)-O-[a-l-rhamnopyranosyl-(1→2)]-a-Larabinopyranosyl)oxy]-30-noroleana-12,20(29)-dien-28-oic acid b-d-glucopyranosyl ester sodium salt | Triterpene glycosides |
| Aerial parts, methanolic extract | Larrealignans A and B | Lignans |
| Leaves, chloroformic extract | dihydroguaiaretic acid, 4-epilarreatricin, 3′-demethoxy-6-Odemethylisoguaiacin, | Lignans |
| Leaves, chloroformic extract | 5,4′-dihydroxy-3,7,8,3′-tetramethoxyflavone 5,4′-dihydroxy-3,7,8-trimethoxyflavone 5,4′-dihydroxy-7-methoxyflavone 5,8,4′-trihydroxy-3,7-dimethoxyflavone | Flavonoids |
| Aerial parts, dichloromethane extract | 3,4-dehydrolarreatricin meso-dihydroguaiaretic acid 3-O-methyldihydroguaiaretic acid 3-O-demethylisoguaiacin | Lignans |
| Aerial parts, dichloromethane extract | 3′-oxohexyl ferulate | Ferulic acid ester |
| Aerial parts, dichloromethane extract | Naringenin 3′-O-methyltaxifolin apigenin-7-methylether Kaempferol-3,7-dimethylether herbacetin-3,7-dimethylether | Flavonoids |
| Leaves, hexane extract | 4,4′-dihydroxy-3-methoxy-6,7′-cyclolignan 3,4-dihydroxy-3′,4′-dimethoxy-6,7′-cyclolignan | Cyclolignans |
| Activities | Bioactive Compounds | Mechanism of Action | Reference |
|---|---|---|---|
| Antioxidant | NDGA, Quercetin, Kaempferol, Justicidin B and Beta peltain | Mitigation of cutaneous lipid peroxidation and cytotoxicity, inhibition of production of hydrogen peroxide and edema formation, reduction of apoptosis hallmarks | [30,31,32,33,34] |
| Antitumor | NDGA | Induction of mitochondrial alterations, ferroptosis. | [35,36] |
| Neuroprotective | NDGA | Promotion of neurogenesis and angiogenesis, anti-apoptotic, reduction of the neurotoxic, motor and cognitive impairments of Alzheimer´s disease | [37,38,39] |
| Regenerative | Not indicated | Inhibition of inflammation or toxicity | [40] |
| Hepatoprotective | NDGA | Lower lipid peroxidation, increase in antioxidant capacity in the liver | [41,42,43] |
| Renal effects | NDGA | Decreasing the activity of renal antioxidant enzymes, affection of mitochondrial activities. | [44,45,46] |
| Anti-inflammatory | NDGA | Reduction in myeloperoxidase activity, reduced edema response, decrease of inflammatory factors | [31,47] |
| Hypoglycemic | NDGA | Inhibition of α-amylase, α-glucosidase and dipeptidyl peptidase 4 | [50] |
| Activities | Bioactive Compound | Mechanism of Action | Reference |
|---|---|---|---|
| Antibacterial activity | Several bioactive Compounds | Affecting proteins of ABC transport system causing bacteria death, bacterial retardation, bacteriostatic, permeabilizing membrane | [12,27,51,52,53,54,55,56,58,59,60,61] |
| Antimycobacterial activity | Lignans, flavonoids, meso-dihydroguaiaretic acid, NDGA | Growth inhibition, bactericidal | [27,55,58,59,64] |
| Antiviral activity | Terameprocol (TMP) | Inhibition of poxvirus growth | [65] |
| Antiprotozoal activity | Several compounds | Modulation of cysteine protease activity present in the trophozoites | [28,66,67] |
| Anthelmintic activity | Hydro methanolic Extracts | Damaging larvae cuticle, coiling up of worms and lethargic movements | [68] |
| Antifungal activity | Tannins, polyphenolic extracts | Fungi-static and fungicidal effects | [31,69,70,71,72,73,74,75,76] |
| Insecticidal activity | Different extracts | Repellent effect, causing death of mosquito larvae | [79,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Madariaga-Navarrete, A.; Higuera-Piedrahita, R.I.; Delgadillo-Ruiz, L.; Bañuelos-Valenzuela, R.; Zaragoza-Bastida, A. Phytochemical Compounds and Pharmacological Properties of Larrea tridentata. Molecules 2022, 27, 5393. https://doi.org/10.3390/molecules27175393
Morales-Ubaldo AL, Rivero-Perez N, Valladares-Carranza B, Madariaga-Navarrete A, Higuera-Piedrahita RI, Delgadillo-Ruiz L, Bañuelos-Valenzuela R, Zaragoza-Bastida A. Phytochemical Compounds and Pharmacological Properties of Larrea tridentata. Molecules. 2022; 27(17):5393. https://doi.org/10.3390/molecules27175393
Chicago/Turabian StyleMorales-Ubaldo, Ana Lizet, Nallely Rivero-Perez, Benjamín Valladares-Carranza, Alfredo Madariaga-Navarrete, Rosa Isabel Higuera-Piedrahita, Lucía Delgadillo-Ruiz, Rómulo Bañuelos-Valenzuela, and Adrian Zaragoza-Bastida. 2022. "Phytochemical Compounds and Pharmacological Properties of Larrea tridentata" Molecules 27, no. 17: 5393. https://doi.org/10.3390/molecules27175393