Bioassay-Guided Isolation of New Flavonoid Glycosides from Platanus × acerifolia Leaves and Their Staphylococcus aureus Inhibitory Effects
Abstract
:1. Introduction
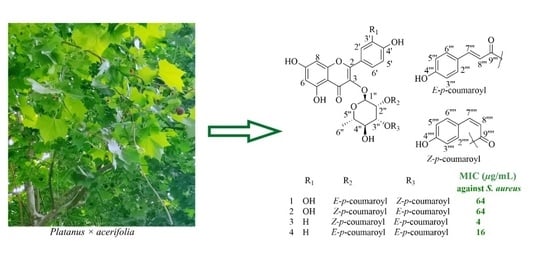
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures and Agents
3.2. Plant Material
3.3. Extraction and Isolation
3.4. In Vitro Antibacterial Susceptibility Assays
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Deurenberg, R.H.; Stobberingh, E.E. The evolution of Staphylococcus aureus. Infect. Genet Evol. 2008, 8, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.P.; Clemons, W.M.; Brodersen, D.E.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 2000, 407, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Sahreena, L.; Zhang, K.J.C.M.R. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar]
- Ibrahim, M.A.; Mansoor, A.A.; Gross, A.; Ashfaq, M.K.; Jacob, M.; Khan, S.I.; Hamann, M.T. Methicillin-resistant Staphylococcus aureus (MRSA)-active metabolites from Platanus occidentalis (American Sycamore). J. Nat. Prod. 2009, 72, 2141–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Liang, M.; Ge, X.; Xua, H.; Ma, K.; Zhang, W.; Zan, Y.; Efferth, T.; Xue, Z.; Hua, X. Phytochemicals with activity against methicillin-resistant Staphylococcus aureus. Phytomedicine 2022, 100, 154073. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Thai, Q.D.; Tchoumtchoua, J.; Mitakou, S.; Halabalaki, M.; Makropoulou, M.; Boulaka, A.; Meligova, A.K.; Mitsiou, D.J.; Alexis, M.N.; Michel, S.; et al. Phytochemical study and biological evaluation of chemical constituents of Platanus orientalis and Platanus × acerifolia buds. Phytochemistry 2016, 130, 170–181. [Google Scholar] [CrossRef]
- Wharton, J.; Izaguirre, I.; Surdock, A.; VandenBerg, M.; Bolhuis, S.; Howard, J.; Muyskens, M. Hands-on demonstration of natural substance fluorescence in simple tree extracts: Sycamore. J. Chem. Educ. 2018, 95, 615–619. [Google Scholar] [CrossRef]
- Haider, S.; Nazreen, S.; Alam, M.M.; Hamid, H.; Alam, M.S. Anti-inflammatory and anti-nociceptive activities of Platanus orientalis Linn. and its ulcerogenic risk evaluation. J. Ethnopharmacol. 2012, 143, 236–240. [Google Scholar] [CrossRef]
- Gilani, S.A. Indigenous knowledge and folk use of medicinal plants by the tribal communities of Hazar Nao Forest, Malakand District, North Pakistan. J. Med. Plant Res. 2011, 5, 1072–1086. [Google Scholar]
- Li, Y.; Wang, S.; Chen, Q. Potential of thirteen urban greening plants to capture particulate matter on leaf surfaces across three levels of ambient atmospheric pollution. Int. J. Environ. Res. Public Health 2019, 16, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, T.; Sun, F.; Song, X.; Zhang, Y.; Huang, F.; Yuan, C.; Yu, H.; Zhang, G.; Qi, F.; et al. The relationship between particulate matter retention capacity and leaf surface micromorphology of ten tree species in Hangzhou, China. Sci. Total Environ. 2021, 771, 144812. [Google Scholar] [CrossRef] [PubMed]
- Kaouadji, M.; Morand, J.M.; Garcia, J. Further acylated kaempferol rhamnosides from Platanus acerifolia buds. J. Nat. Prod. 1993, 56, 1618–1621. [Google Scholar] [CrossRef]
- Kaouadji, M.; Ravanel, P.; Tissut, M.; Creuzet, S. Novel methylated flavonols with unsubstituted B ring from Platanus acerifolia buds. J. Nat. Prod. 1988, 51, 353–356. [Google Scholar] [CrossRef]
- Kaouadji, M.; Ravanel, P.; Mariotte, A.M. New prenylated flavanones from Platanus acerifolia buds. J. Nat. Prod. 1986, 49, 153–155. [Google Scholar] [CrossRef]
- Kaouadji, M.; Morand, J.M.; Gilly, C. 4-Hydroxygrenoblone, another uncommon C-prenylated flavonoid from Platanus acerifolia buds. J. Nat. Prod. 1986, 49, 508–510. [Google Scholar] [CrossRef]
- Kaouadji, M.; Champavier, Y.; Morand, J.M. Platanus acerifolia’s buds: Additional non-polar metabolites and structure revision of two previous dihydrochalcone-like metabolites. Tetrahedron Lett. 2013, 54, 6352–6357. [Google Scholar] [CrossRef]
- Zuo, B.; Liao, Z.; Xu, C.; Liu, C. Two novel prenylated kaempferol derivatives from fresh bud’s fur of Platanus acerifolia and their anti-proliferative activities. Nat. Prod. Res. 2016, 30, 2523–2528. [Google Scholar] [CrossRef]
- Kaouadji, M. Further prenylated flavonols from Platanus acerifolia’s unripe buds. Tetrahedron Lett. 2014, 55, 1285–1288. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; Liu, J. Platachromone A–D: Cytotoxic 2-styrylchromones from the bark of Platanus × acerifolia (Aiton) Willd. Phytochem. Lett. 2013, 6, 387–391. [Google Scholar] [CrossRef]
- Yang, N.; Yu, L.; Shang, E.; Duan, J. Chemical constituents with immunological activities from the barks of Platanus acerifolia. Chem. Nat. Compd. 2014, 50, 384–386. [Google Scholar] [CrossRef]
- Bruckner, V.; Kovács, J.; Koczka, I. Occurrence of betulinic acid in the bark of the plane tree. J. Chem. Soc. 1948, 181, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, G. Isolation and crystal structure of 3β-acetoxy-20(29)-lupen-28-aldehyde from the bark of Platanus acerifolia Willd. Chin. J. Struc. Chem. 2012, 31, 1140–1144. [Google Scholar]
- Pinilla, J.M.; López-Padilla, A.; Vicente, G.; Fornari, T.; Quintela, J.C.; Reglero, G. Recovery of betulinic acid from plane tree (Platanus acerifolia L.). J. Supercrit. Fluid 2014, 95, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Huang, R.; Zuo, B.; Ji, L.; Mo, Z.; Liao, Z. Anti-proliferative activities of two flavonols with unsubstituted B-ring from the leaves of Platanus acerifolia. Nat. Prod. Commun. 2017, 12, 1701–1704. [Google Scholar] [CrossRef]
- Tu, F.; Dai, Y.; Yao, Z.; Wang, X.; Yao, X.; Qin, L. Flavonol glycosides from Epimedium pubescens. Chem. Pharm. Bull. 2011, 59, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Flores, S.E.; Herrán, J. The structure of pendulin and penduletin: A new flavonol glucoside isolated from Brickelia pendula. Tetrahedron 1958, 2, 308–315. [Google Scholar] [CrossRef]
- Özbek, H.; Güvenalp, Z.; Kuruüzüm-Uz, A.E.; Kazaz, C.; Demirezer, L.M. β-Hydroxydihydrochalcone and flavonoid glycosides along with triterpene saponin and sesquiterpene from the herbs of Pimpinella rhodantha Boiss. Nat. Prod. Res. 2016, 30, 750–754. [Google Scholar] [CrossRef]
- Onodera, K.; Hanashiro, K.; Yasumoto, T. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica. Biosci. Biotechnol. Biochem. 2006, 70, 1995–1998. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Pan, G.; Xu, W.; Lu, X.; Bai, C.; Liu, M.; Chen, Y. Isolation and structure elucidation of a new flavonol glycoside from Sabia Parviflora. Nat. Prod. Res. 2021, 35, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Dempster, N.M.; Sarker, S.D. Justicialosides A and B, two new flavone glycosides from the leaves of Ruspolia hypocrateriformis (Vahl) Milne-Redh. (Acanthaceae). Phytochem. Lett. 2019, 31, 101–103. [Google Scholar] [CrossRef]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.; Chen, Y.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]


| Position | δC | δH, Multi. (J in Hz) | Position | δC | δH, Multi. (J in Hz) |
|---|---|---|---|---|---|
| Flavonol moiety | Rhamnosyl moiety | ||||
| 2 | 161.45 | 1″ | 100.35 | 5.53, d (1.6) | |
| 3 | 135.56 | 2″ | 70.80 | 5.81, dd (1.6, 3.6) | |
| 4 | 179.35 | 3″ | 72.83 | 5.25, dd (3.6, 9.6) | |
| 5 | 163.25 | 4″ | 70.91 | 3.58, t (9.6) | |
| 6 | 99.89 | 6.21, d (2.0) | 5″ | 72.27 | 3.60, m |
| 7 | 165.92 | 6″ | 17.74 | 1.03, d (5.2) | |
| 8 | 94.75 | 6.39, d (2.0) | 1‴ | 127.08 | |
| 9 | 158.55 | 2‴, 6‴ | 131.40 | 7.47, br d (8.4) | |
| 10 | 105.88 | 3‴, 5‴ | 116.84 | 6.80, br d (8.4) | |
| 1′ | 122.76 | 4‴ | 159.29 | ||
| 2′ | 116.69 | 7.38, d (2.0) | 7‴ | 147.57 | 7.61, d (15.6) |
| 3′ | 146.61 | 8‴ | 114.29 | 6.31, d (15.6) | |
| 4′ | 149.96 | 9‴ | 167.77 | ||
| 5′ | 116.63 | 6.96, d (8.4) | 1′′′′ | 127.58 | |
| 6′ | 123.05 | 7.42, dd (2.0, 8.4) | 2′′′′, 6′′′′ | 133.85 | 7.69, br d (8.4) |
| 3′′′′, 5′′′′ | 115.87 | 6.77, br d (8.4) | |||
| 4′′′′ | 160.11 | ||||
| 7′′′′ | 145.84 | 6.88, d (12.8) | |||
| 8′′′′ | 116.20 | 5.73, d (12.8) | |||
| 9′′′′ | 167.58 | ||||
| Position | δC | δH, Multi. (J in Hz) | Position | δC | δH, Multi. (J in Hz) |
|---|---|---|---|---|---|
| Flavonol moiety | Rhamnosyl moiety | ||||
| 2 | 161.31 | 1″ | 100.49 | 5.49, d (1.6) | |
| 3 | 135.65 | 2″ | 70.62 | 5.81, dd (1.6, 3.6) | |
| 4 | 179.38 | 3″ | 73.11 | 5.25, dd (3.6, 9.6) | |
| 5 | 163.98 | 4″ | 70.91 | 3.51, t (9.6) | |
| 6 | 99.90 | 6.22, d (2.0) | 5″ | 72.22 | 3.61, m |
| 7 | 165.34 | 6″ | 17.69 | 1.01, d (6.0) | |
| 8 | 94.76 | 6.40, d (2.0) | 1‴ | 127.37 | |
| 9 | 158.56 | 2‴, 6‴ | 133.98 | 7.63, br d (8.8) | |
| 10 | 105.53 | 3‴, 5‴ | 115.99 | 6.63, br d (8.8) | |
| 1′ | 122.73 | 4‴ | 160.22 | ||
| 2′ | 116.68 | 7.39, d (2.0) | 7‴ | 146.58 | 6.92, d (12.8) |
| 3′ | 146.61 | 8‴ | 115.37 | 5.79, d (12.8) | |
| 4′ | 149.97 | 9‴ | 166.58 | ||
| 5′ | 116.61 | 6.97, d (8.4) | 1′′′′ | 127.10 | |
| 6′ | 123.02 | 7.43, dd (2.0, 8.4) | 2′′′′, 6′′′′ | 131.24 | 7.36, br d (8.8) |
| 3′′′′, 5′′′′ | 116.83 | 6.77, br d (8.8) | |||
| 4′′′′ | 160.47 | ||||
| 7′′′′ | 147.10 | 7.63, d (16.0) | |||
| 8′′′′ | 114.94 | 6.28, d (16.0) | |||
| 9′′′′ | 168.65 | ||||
| Compound | MIC (μg/mL) |
|---|---|
| 1 | 64 a |
| 2 | 64 a |
| 3 | 4 c |
| 4 | 16 b |
| Methicillin | 2 d |
| Chloramphenicol | 4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Tang, Y.; Osman, E.E.A.; Wan, J.; Jiang, W.; Yang, G.; Xiong, J.; Zhu, Q.; Hu, J.-F. Bioassay-Guided Isolation of New Flavonoid Glycosides from Platanus × acerifolia Leaves and Their Staphylococcus aureus Inhibitory Effects. Molecules 2022, 27, 5357. https://doi.org/10.3390/molecules27175357
Wu X, Tang Y, Osman EEA, Wan J, Jiang W, Yang G, Xiong J, Zhu Q, Hu J-F. Bioassay-Guided Isolation of New Flavonoid Glycosides from Platanus × acerifolia Leaves and Their Staphylococcus aureus Inhibitory Effects. Molecules. 2022; 27(17):5357. https://doi.org/10.3390/molecules27175357
Chicago/Turabian StyleWu, Xiying, Yu Tang, Ezzat E. A. Osman, Jiang Wan, Wei Jiang, Guoxun Yang, Juan Xiong, Quangang Zhu, and Jin-Feng Hu. 2022. "Bioassay-Guided Isolation of New Flavonoid Glycosides from Platanus × acerifolia Leaves and Their Staphylococcus aureus Inhibitory Effects" Molecules 27, no. 17: 5357. https://doi.org/10.3390/molecules27175357