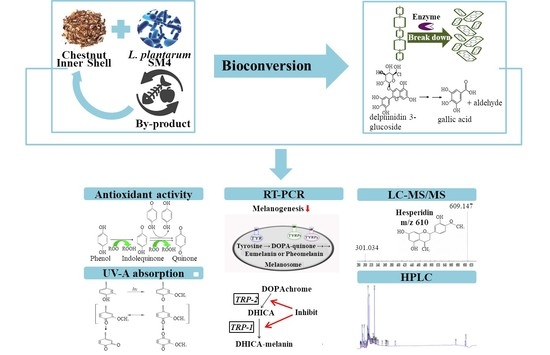
Enhancement of TRP Gene Expression and UV Absorption by Bioconverted Chestnut Inner Shell Extracts Using Lactiplantibacillus plantarum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Measurement of Antioxidant Activity
2.2. Measurement of Tyrosinase Activity Inhibition and UV-A Absorption
2.3. Measurement of Cell Viability
2.4. Measurement of Gene Expression Levels
2.5. LC-MS/MS Analysis
2.6. HPLC Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Ultrasound-Assisted Extraction
3.3. Isolation and Characterization of Microorganism
3.4. Bioconversion
3.5. Measurement of Total Polyphenols, Total Flavonoids, and Antioxidant Activity
3.6. Measurement of Tyrosinase Activity Inhibition and UV-A Absorption
3.7. Cell Viability Assay
3.8. Measurement of Gene Expression
3.9. Measurement of Main Components
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Stefan, C. The Left-Over Problem: The Blind Spot of the Automotive Portion of the Advanced Energy Intiative. Sustain. Dev. Law Policy 2010, 7, 10. [Google Scholar]
- Crutzen, P.J.; Ehhalt, D.H. Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer. Ambio 1977, 6, 112–117. [Google Scholar]
- Herndon, J.M.; Hoisington, R.D.; Whiteside, M. Deadly ultraviolet UV-C and UV-B penetration to Earth’s surface: Human and environmental health implications. J. Geogr. Environ. Earth Sci. Int. 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Damiani, E.; Baschong, W.; Greci, L. UV-Filter combinations under UV-A exposure: Concomitant quantification of over-all spectral stability and molecular integrity. J. Photochem. Photobiol. B Biol. 2007, 87, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Pen, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Empson, Y.M.; Ekwueme, E.C.; Hong, J.K.; Paynter, D.M.; Kwansa, A.L.; Brown, C.; Pekkanen, A.M.; Roman, M.; Rylander, N.M.; Brolinson, G.P.; et al. High elastic modulus nanoparticles: A novel tool for subfailure connective tissue matrix damage. Transl. Res. 2014, 164, 244–257. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules 2016, 21, 70. [Google Scholar] [CrossRef] [Green Version]
- Usuki, A.; Ohashi, A.; Sato, H.; Ochiai, Y.; Ichihashi, M.; Funasaka, Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp. Dermatol. 2003, 12, 43–50. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, H.K.; Lee, K.J.; Jeon, H.W.; Cui, S.; Lee, Y.M.; Moon, B.J.; Kim, Y.H.; Lee, Y.S. Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB Rep. 2010, 43, 461–467. [Google Scholar] [CrossRef]
- Soares, A.R.; de Lourdes Lucio Ferrarese, M.; de Cássia Siqueira-Soares, R.; Marchiosi, R.; Finger-Teixeira, A.; Ferrarese-Filho, O. The allelochemical L-DOPA increases melanin production and reduces reactive oxygen species in soybean roots. J. Chem. Ecol. 2011, 37, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Cinici, E.; Dilekmen, N.; Kutlu, Z.; Dincer, B.; Cinici, O.; Balta, H.; Calık, I. Carvone protects against paclitaxel-induced retinal and optic nerve cytotoxicity: A histopathological study. Cutan. Ocul. Toxicol. 2019, 38, 290–293. [Google Scholar] [CrossRef]
- Pathak, S.; Chaudhary, H.S. Perspective of microbial species used in lignocelluloses bioconversion. Cellulose 2013, 35, 50. [Google Scholar]
- Verma, D.; Singla, A.; Lal, B.; Sarma, P.M. Conversion of biomass-generated syngas into next-generation liquid transport fuels through microbial intervention: Potential and current status. Curr. Sci. 2016, 110, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.Y.; Joo, S.Y. Antioxidant activity and quality characteristics of cookies with chestnut Inner shell. Korean J. Food Nutr. 2012, 25, 224–232. [Google Scholar]
- Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, Y.; Ando, H.; Murata, Y.; Shimoishi, Y.; Tada, M.; Takahata, K. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci. Biotechnol. Biochem. 2003, 67, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.Y. Effects of lactic acid fermentation of bamboo shoot sheath on cosmetics usefulness. Journal Korean Soc. Cosmetol. 2020, 26, 321–332. [Google Scholar]
- Hernández-Hierro, J.M.; Quijada-Morín, N.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Relationship between skin cell wall composition and anthocyanin extractability of Vitis vinifera L. cv. Tempranillo at different grape ripeness degree. Food Chem. 2014, 146, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulski, D.; Molski, M. A quantum chemical study on the antioxidant activity of bioactive polyphenols from peanut (Arachis hypogaea) and the major metabolites of trans-resveratrol. Comput. Theor. Chem. 2012, 981, 38–46. [Google Scholar] [CrossRef]
- Sun, M.F.; Jiang, C.L.; Kong, Y.S.; Luo, J.L.; Yin, P.; Guo, G.Y. Recent Advances in Analytical Methods for Determination of Polyphenols in Tea: A Comprehensive Review. Foods 2022, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, K.H.; Yook, H.S. Antioxidant Activities of Fermented Sophorae fructus, and Inhibitiory Actions on Tyrosinase and Elastase. J. Korean Soc. Food Sci. Nutr. 2021, 50, 254–263. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Botanical antioxidants in the prevention of photocarcinogenesis and photoaging. Exp. Dermatol. 2006, 15, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Hridya, H.; Amrita, A.; Sankari, M.; Doss, C.G.P.; Gopalakrishnan, M.; Gopalakrishnan, C.; Siva, R. Inhibitory effect of brazilein on tyrosinase and melanin synthesis: Kinetics and in silico approach. Int. J. Biol. Macromol. 2015, 81, 228–234. [Google Scholar] [CrossRef]
- Sim, J.H.; Lee, K.M.; Park, T.J.; Kang, M.S.; Hong, H.H.; Kim, S.Y. Biorenovation-assisted modifiction of Ligustrum japonicum extract for skin-whitening effect. Korean Soc. Biotechnol. Bioeng. J. 2021, 36, 30–35. [Google Scholar]
- Daduang, J.; Palasap, A.; Daduang, S.; Boonsiri, P.; Suwannalert, P.; Limpaiboon, T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, T.R.; Lin, J.J.; Tsai, C.C.; Huang, T.K.; Yang, Z.Y.; Wu, M.O.; Zheng, Y.Q.; Su, C.C.; Wu, Y.J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Kang, M.K.; Kim, Y.H.; Kim, D.Y.; Oh, H.; Kim, S.I.; Na, W.; Kang, Y.H. Coumarin ameliorates impaired bone turnover by inhibiting the formation of advanced glycation end products in diabetic osteoblasts and osteoclasts. Biomolecules 2020, 10, 1052. [Google Scholar] [CrossRef]
- Masamoto, Y.; Murata, Y.; Baba, K.; Shimoishi, Y.; Tada, M.; Takahata, K. Inhibitory effects of esculetin on melanin biosynthesis. Biol. Pharm. Bull. 2004, 27, 422–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.J.; Chung, T.W.; Lee, J.H.; Kim, B.S.; Kim, E.Y.; Lee, S.O.; Ha, K.T. Water-extracted branch of Cinnamomum cassia promotes lung cancer cell apoptosis by inhibiting pyruvate dehydrogenase kinase activity. J. Pharmacol. Sci. 2018, 138, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J. Agric. Food Chem. 2002, 50, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Agrawal, P.; Rajput, S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Kubo, I.; Kinst-Hori, I. Flavonols from saffron flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Shadboorestan, A. Oxidative stress and cancer; the role of hesperidin, a citrus natural bioflavonoid, as a cancer chemoprotective agent. Nutr. Cancer 2016, 68, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Stanisic, D.; Liu, L.H.; Dos Santos, R.V.; Costa, A.F.; Durán, N.; Tasic, L. New sustainable process for hesperidin isolation and anti-ageing effects of hesperidin nanocrystals. Molecules 2020, 25, 4535. [Google Scholar] [CrossRef]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Modulatory effect of hesperidin on benzo (a) pyrene induced experimental lung carcinogenesis with reference to COX-2, MMP-2 and MMP-9. Eur. J. Pharmacol. 2010, 649, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Gogna, N.; Hamid, N.; Dorai, K. Metabolomic profiling of the phytomedicinal constituents of Carica papaya L. leaves and seeds by 1H NMR spectroscopy and multivariate statistical analysis. J. Pharm. Biomed. Anal. 2015, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Dietz, B.M.; Kang, Y.H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; Breemen, R.B.V. Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, A.R.; Kim, M.J.; Park, S.N. Antibacterial, antioxidative and antiaging effects of Allium cepa peel extracts. Appl. Chem. Eng. 2011, 22, 178–184. [Google Scholar]
- Khosravi, A.; Razavi, S.H. Therapeutic effects of polyphenols in fermented soybean and black soybean products. J. Funct. Foods 2021, 81, 104469. [Google Scholar] [CrossRef]







| Genes | Forward Primers (5′-3′) | Reverse Primers (5′-3′) | Size (bp) |
|---|---|---|---|
| 1TRP-1 | GCTGCAGGAGCC TTCTTTCTC | AAGACGCTGCACTGCTGGTCT | 398 |
| 2TRP-2 | AGAAGTTTGACAGCCCTCC | CTAACCGCAGAGCAACTTG | 360 |
| β-actin | ACTACCTCATGAAGATCCTG | TTGCTGATCCACATCTGCTG | 731 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Yoem, S.-H.; Kim, J.-H.; Hong, J.-W.; Oh, Y.-S.; Kim, J.-W. Enhancement of TRP Gene Expression and UV Absorption by Bioconverted Chestnut Inner Shell Extracts Using Lactiplantibacillus plantarum. Molecules 2022, 27, 4940. https://doi.org/10.3390/molecules27154940
Kim S-H, Yoem S-H, Kim J-H, Hong J-W, Oh Y-S, Kim J-W. Enhancement of TRP Gene Expression and UV Absorption by Bioconverted Chestnut Inner Shell Extracts Using Lactiplantibacillus plantarum. Molecules. 2022; 27(15):4940. https://doi.org/10.3390/molecules27154940
Chicago/Turabian StyleKim, So-Hee, Suh-Hee Yoem, Jun-Hee Kim, Ji-Woo Hong, Ye-Sol Oh, and Jin-Woo Kim. 2022. "Enhancement of TRP Gene Expression and UV Absorption by Bioconverted Chestnut Inner Shell Extracts Using Lactiplantibacillus plantarum" Molecules 27, no. 15: 4940. https://doi.org/10.3390/molecules27154940