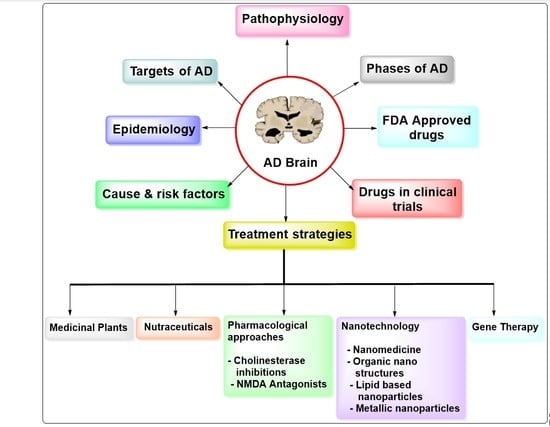
Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology
Abstract
:1. Introduction
1.1. Phases of Alzheimer’s Disease
1.2. Cause and Risk Factors of Alzheimer’s Disease
1.2.1. Health and Lifestyle Factors
1.2.2. Environmental Factors
1.2.3. Genetic Factors
2. Epidemiology
3. Pathophysiology
3.1. Hyperphosphorylated Tau Protein and Aβ Hypothesis
3.2. Oxidative Stress Conditions
3.3. Unbalance of Metal Ions
3.4. Cholinergic Theory
3.5. Amyloid Cascade Theory
4. Targets of Alzheimer’s Disease
4.1. Acetylcholinesterase
4.2. NMDA Receptor
4.3. Glycogen Synthase Kinase (GSK3)
4.4. BACE1
5. Evolution of Treatment Strategies
5.1. Medicinal Plants Used in the Treatment of AD
5.2. Nutraceuticals
5.2.1. Aged Garlic Extract (AGE)
5.2.2. Alpha-Lipoic Acid
5.2.3. Caffeine and Chlorogenic Acids
5.2.4. Caprylic Acid
5.2.5. Cinnamon
5.2.6. Curcumin
5.2.7. Omega-3 Fatty Acid and Fish Liver Oil
5.2.8. Grape Seed Extract (GSE)
5.2.9. Piperine
5.2.10. Resveratrol
5.2.11. Quercetin
5.3. Pharmacological Approaches
5.3.1. Cholinergic Inhibitors
Donepezil
Rivastigmine
Galantamine (GAL)
5.3.2. NMDA Antagonists
5.4. Nanotechnology and Treatment of AD
5.4.1. Nanomedicine
5.4.2. Organic Nanostructures
Polymeric-Based NPs
Nanomicellar
Dendrimers
Nanogels
5.4.3. Lipid-Based Nanoparticles
Solid Lipid NPs
Liposomes
Niosomes
Nanoemulsion
Cubosomes
Amylolipid Nanovesicles
5.4.4. Metallic Nanoparticles
Selenium NPs
Cerium NPs
Gold NPs
Iron NPs
Green Synthesized Nanoparticles
5.5. Gene Therapy
Mechanism of Antisense Drug Delivery to the Brain
6. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | adeno-associated viruses |
| Ab | antibody |
| ACh | acetylcholine |
| AChE | acetylcholinesterase |
| AChEIs | acetyl-cholinesterase inhibitors |
| AGE | aged garlic extract |
| AD | Alzheimer’s disease |
| APP | amyloid precursor protein |
| Aβ | amyloid-beta |
| AS-On’s | antisense oligonucleotides |
| APOE | apo-lipo-protein E |
| ApoE2 | apolipoprotein E |
| BACE1 | β-site APP cleaving enzyme 1 |
| BBB | blood–brain barrier |
| BMP-9 | bone morphogenetic protein |
| BuChE | butyrylcholinesterase |
| CA1 | cornu Ammonis1 |
| cAMP | cyclic adenosine monophosphate |
| CAT | choline acetyl transferase |
| CTF | carboxyl-terminal fragment |
| CPPs | cell-penetrating peptides |
| CeONPs | cerium oxide NPs |
| cGMP | cyclic guanosine monophosphate |
| CHT1 | choline transporter |
| ChEIs | cholinesterase inhibitors |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DNA | deoxyribonucleic acid |
| EGCG | epigallocation-3gallate |
| FA: | fatty Acid |
| FAD | familial AD |
| FDA | Food and Drug Administration |
| GAL | galantamine |
| GSK3 | glycogen synthase kinase 3 |
| GSE | grape seed extract |
| HIV | human immunodeficiency virus |
| LTP | long-term potentiation |
| MA | monoclonal antibody |
| MLV | murine leukemia virus |
| NFT | neurofibrillary tangle |
| NMDA | N-methyl D-aspartate |
| NP | nanoparticles |
| Ost | osthole |
| PC12 | adrenal phaeochromocytoma cell line |
| PEG | polyethylene glycol |
| PNAs | peptide nucleic acids |
| PDEs | phosphodiesterases |
| PI3K | phosphoinositide 3-kinase |
| PLGA | poly lactic-co-glycolic acid |
| PSEN1 | presenilin 1 |
| PSEN2 | presenilin 2 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SP | senile plaques |
| STZ | streptozotocin |
| TLC | thin layer chromatography |
References
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Prasansuklab, A.; Tencomnao, T. Amyloidosis in Alzheimer’s disease: The Toxicity of Amyloid Beta (Aβ), Mechanisms of Its Accumulation and Implications of Medicinal Plants for Therapy. Evid.-Based Complement. Altern. Med. 2013, 2013, 413808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, T.D. Early-onset familial Alzheimer’s disease. In GeneReviews; University of Washington: Seattle, WA, USA, 2012; pp. 1–42. [Google Scholar]
- Chu, L.W. Alzheimer’s disease: Early diagnosis and treatment. Hong Kong Med. J. 2012, 18, 228–237. [Google Scholar] [PubMed]
- Sun, X.; Chen, W.-D.; Wang, Y.-D. β-Amyloid: The key peptide in the pathogenesis of Alzheimer’s disease. Front. Pharmacol. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervellati, C.; Wood, P.L.; Romani, A.; Valacchi, G.; Squerzanti, M.; Sanz, J.M.; Ortolani, B.; Zuliani, G. Oxidative challenge in Alzheimer’s disease: State of knowledge and future needs. J. Investig. Med. 2016, 64, 21–32. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipton, S.A.; Nicotera, P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium 1998, 23, 165–171. [Google Scholar] [CrossRef]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2018, 29, 125–133. [Google Scholar] [CrossRef]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Donepezil and Memantine for Moderate-to-Severe Alzheimer’s disease. N. Engl. J. Med. 2012, 366, 893–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farlow, M.; Anand, R.; Messina, J., Jr.; Hartman, R.; Veach, J. A 52-Week Study of the Efficacy of Rivastigmine in Patients with Mild to Moderately Severe Alzheimer’s disease. Eur. Neurol. 2000, 44, 236–241. [Google Scholar] [CrossRef]
- Tripathi, A.C.; Saraf, S.A.; Saraf, S.K.; Tripathi, A.C.; Saraf, S.A.; Saraf, S.K. Carbon Nanotropes: A Contemporary Paradigm in Drug Delivery. Materials 2015, 8, 3068–3100. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Mortsdorf, T.; Ritter, A.; Zhong, K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2017, 3, 367–384. [Google Scholar] [CrossRef]
- Rayathala, J.; Kumar, K.; Venkatesh, P. Review on Alzheimer’s disease: Past, present and future. J. Innov. Appl. Pharm. Sci. 2022, 7, 28–31. [Google Scholar] [CrossRef]
- Cipriani, G.; Dolciotti, C.; Picchi, L.; Bonuccelli, U. Alzheimer and his disease: A brief history. Neurol. Sci. 2010, 32, 275–279. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 8 December 2020).
- Wattmo, C.; Minthon, L.; Wallin, K. Mild versus moderate stages of Alzheimer’s disease: Three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy. Alzheimer’s Res. Ther. 2016, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Apostolova, L.G. Alzheimer Disease. Contin. Lifelong Learn. Neurol. 2016, 22, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Blass, J.P. Alzheimer’s disease. Disease-a-Month 1985, 31, 8–69. [Google Scholar] [CrossRef]
- Terry, R.D.; Davies, P. Dementia of the Alzheimer Type. Annu. Rev. Neurosci. 1980, 3, 77–95. [Google Scholar] [CrossRef]
- Rathmann, K.L.; Conner, C.S. Alzheimer’s disease: Clinical Features, Pathogenesis, and Treatment. Ann. Pharmacother. 2007, 41, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer’s disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Imtiaz, B.; Tolppanen, A.-M.; Kivipelto, M.; Soininen, H. Future directions in Alzheimer’s disease from risk factors to prevention. Biochem. Pharmacol. 2014, 88, 661–670. [Google Scholar] [CrossRef] [PubMed]
- González-Maciel, A.; Reynoso-Robles, R.; Torres-Jardón, R.; Mukherjee, P.S.; Calderón-Garcidueñas, L. Combustion-Derived Nanoparticles in Key Brain Target Cells and Organelles in Young Urbanites: Culprit Hidden in Plain Sight in Alzheimer’s disease Development. J. Alzheimer’s Dis. 2017, 59, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reitz, C.; Mayeux, R. Alzheimer’s disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikhonova, M.A.; Chang, H.M.; Singh, S.K.; Vieau, D. Experimental and Innovative Approaches to Multi-Target Treatment of Parkinson’s and Alzheimer’s diseases. Front. Neurosci. 2022, 16, 910020. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; Wilson, R.S.; Evans, D.A. Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimer’s Dement. 2018, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef]
- Montgomery, W.; Ueda, K.; Jorgensen, M.; Stathis, S.; Cheng, Y.; Nakamura, T. Epidemiology, associated burden, and current clinical practice for the diagnosis and management of Alzheimer’s disease in Japan. Clin. Outcomes Res. 2018, 10, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The Prevalence and Incidence of Dementia Due to Alzheimer’s disease: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2016, 43 (Suppl. 1), S51–S82. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, C.; Thompson, P.L.; van Walsem, A.; Faure, C.; Maier, W.C. Epidemiological and Economic Burden of Alzheimer’s disease: A Systematic Literature Review of Data across Europe and the United States of America. J. Alzheimer’s Dis. 2015, 43, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hou, N.-N.; Wu, H.-M.; Zuo, X.; Lian, Y.-Z.; Zhang, C.-N.; Wang, Z.-F.; Zhang, X.; Zhu, J.-H. Prevalence of Alzheimer’s disease and Parkinson’s Disease in China: An Updated Systematical Analysis. Front. Aging Neurosci. 2020, 12, 603854. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X. The prevalence of Alzheimer’s disease in the Chinese Han population: A meta-analysis. Neurol. Res. 2020, 42, 291–298. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wang, W.; Wu, J.J.; Liu, L.; Theodoratou, E.; Car, J.; Middleton, L.; Russ, T.C.; Deary, I.J.; Campbell, H.; et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet 2013, 381, 2016–2023. [Google Scholar] [CrossRef]
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Al-Rahamneh, M.J.; Aguinaga-Ontoso, I. Trends of mortality from Alzheimer’s disease in the European Union, 1994–2013. Eur. J. Neurol. 2017, 24, 858–866. [Google Scholar] [CrossRef]
- Park, J. Mortality from Alzheimer’s disease in Canada: A multiple-cause-of-death analysis, 2004 to 2011. Health Rep. 2016, 27, 17–21. [Google Scholar]
- Kramarow, E.A.; Tejada-Vera, B. Dementia Mortality in the United States, 2000–2017. Natl. Vital Stat. Rep. Cent. Dis. Control Prev. Natl. Cent. Health Stat. Natl. Vital Stat. Syst. 2019, 68, 1–29. [Google Scholar]
- Price, A.; Farooq, R.; Yuan, J.-M.; Menon, V.B.; Cardinal, R.; O’Brien, J. Mortality in dementia with Lewy bodies compared with Alzheimer’s dementia: A retrospective naturalistic cohort study. BMJ Open 2017, 7, e017504. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75.e2. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kametani, F.; Hasegawa, M. Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer’s disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, A.; Sun, H.; Han, Y.; Kong, L.; Wang, X. Two decades of new drug discovery and development for Alzheimer’s disease. RSC Adv. 2017, 7, 6046–6058. [Google Scholar] [CrossRef] [Green Version]
- Prakash, A.; Dhaliwal, G.K.; Kumar, P.; Majeed, A.B.A. Brain biometals and Alzheimer’s disease—Boon or bane? Int. J. Neurosci. 2016, 127, 99–108. [Google Scholar] [CrossRef]
- Sultzer, D.L.; Marder, S.R. Older Brains are Different: Brain–Behavior Studies and Their Clinical Utility. Am. J. Geriatr. Psychiatry 2017, 25, 11–12. [Google Scholar] [CrossRef]
- Chase, T.N.; Farlow, M.R.; Clarence-Smith, K. Donepezil Plus Solifenacin (CPC-201) Treatment for Alzheimer’s disease. Neurotherapeutics 2017, 14, 405–416. [Google Scholar] [CrossRef] [Green Version]
- Lane, R.M.; Kivipelto, M.; Greig, N.H. Acetylcholinesterase and Its Inhibition in Alzheimer’s disease. Clin. Neuropharmacol. 2004, 27, 141–149. [Google Scholar] [CrossRef]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today 2003, 39, 75–83. [Google Scholar] [CrossRef]
- Alvarez, A.; Alarcón, R.; Opazo, C.; Campos, E.O.; Muñoz, F.J.; Calderón, F.H.; Dajas, F.; Gentry, M.K.; Doctor, B.P.; De Mello, F.G.; et al. Stable Complexes Involving Acetylcholinesterase and Amyloid-β Peptide Change the Biochemical Properties of the Enzyme and Increase the Neurotoxicity of Alzheimer’s Fibrils. J. Neurosci. 1998, 18, 3213–3223. [Google Scholar] [CrossRef] [Green Version]
- Radic, Z.; Pickering, N.A.; Vellom, D.C.; Camp, S.; Taylor, P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry 1993, 32, 12074–12084. [Google Scholar] [CrossRef]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Vyklicky, V.; Korinek, M.; Smejkalova, T.; Balik, A.; Krausova, B.; Kaniakova, M.; Lichnerova, K.; Černý, J.; Krusek, J.; Dittert, I.; et al. Structure, Function, and Pharmacology of NMDA Receptor Channels. Physiol. Res. 2014, 63, S191–S203. [Google Scholar] [CrossRef]
- Foster, T.; Kyritsopoulos, C.; Kumar, A. Central role for NMDA receptors in redox mediated impairment of synaptic function during aging and Alzheimer’s disease. Behav. Brain Res. 2017, 322, 223–232. [Google Scholar] [CrossRef]
- Ito, K.; Tatebe, T.; Suzuki, K.; Hirayama, T.; Hayakawa, M.; Kubo, H.; Tomita, T.; Makino, M. Memantine reduces the production of amyloid-β peptides through modulation of amyloid precursor protein trafficking. Eur. J. Pharmacol. 2017, 798, 16–25. [Google Scholar] [CrossRef]
- Kumar, G.; Patnaik, R. Exploring neuroprotective potential of Withania somnifera phytochemicals by inhibition of GluN2B-containing NMDA receptors: An in silico study. Med. Hypotheses 2016, 92, 35–43. [Google Scholar] [CrossRef]
- Kang, Q.; Xiang, Y.; Li, D.; Liang, J.; Zhang, X.; Zhou, F.; Qiao, M.; Nie, Y.; He, Y.; Cheng, J.; et al. MiR-124-3p attenuates hyperphosphorylation of tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells. Oncotarget 2017, 8, 24314–24326. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal Hyperphosphorylation of Tau: Sites, Regulation, and Molecular Mechanism of Neurofibrillary Degeneration. J. Alzheimer’s Dis. 2013, 33 (Suppl. 1), S123–S139. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsieh, Y.-S.; Wu, Y.-R.; Hsu, C.-J.; Chen, H.-C.; Huang, W.-H.; Chang, K.-H.; Hsieh-Li, H.M.; Su, M.-T.; Sun, Y.-C.; et al. Identifying GSK-3β kinase inhibitors of Alzheimer’s disease: Virtual screening, enzyme, and cell assays. Eur. J. Pharm. Sci. 2016, 89, 11–19. [Google Scholar] [CrossRef]
- Coimbra, J.; Marques, D.F.F.; Baptista, S.J.; Pereira, C.M.F.; Moreira, P.I.; Dinis, T.C.P.; Santos, A.E.; Salvador, J.A.R. Highlights in BACE1 Inhibitors for Alzheimer’s disease Treatment. Front. Chem. 2018, 6, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The β-Secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Vassar, R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Hamada, Y.; Nguyen, J.-T.; Kiso, Y. Novel BACE1 inhibitors with a non-acidic heterocycle at the P1′ position. Bioorg. Med. Chem. 2013, 21, 6665–6673. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, H.; Li, C.; Wang, D.; Wu, J.; Wang, C.; Xu, J.; Qin, R.-A. Cognitive improvement of compound danshen in an Aβ25-35 peptide-induced rat model of Alzheimer’s disease. BMC Complement. Altern. Med. 2015, 15, 382. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.H.V.; Gupta, Y.K. Effect of Centella asiatica on cognition and oxidative stress in an intracerebroventricular streptozotocin model of Alzheimer’s disease in rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 336–342. [Google Scholar] [CrossRef]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin Structure-Function, Bioavailability, and Efficacy in Models of Neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [Green Version]
- Bihaqi, S.W.; Sharma, M.; Singh, A.P.; Tiwari, M. Neuroprotective role of Convolvulus pluricaulis on aluminium induced neurotoxicity in rat brain. J. Ethnopharmacol. 2009, 124, 409–415. [Google Scholar] [CrossRef]
- Mokhtarian, A.; Esfandiari, E.; Ghanadian, M.; Rashidi, B.; Vatankhah, A.M. The effects of Acorus calamus L. in preventing memory loss, anxiety, and oxidative stress on lipopolysaccharide-induced neuroinflammation rat models. Int. J. Prev. Med. 2018, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Houghton, P.; Whang, W.; Cho, J. Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 2004, 11, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.B.; Lahiri, D.K. The “Aged Garlic Extract” (AGE) and one of its Active Ingredients S-Allyl-LCysteine (SAC) as Potential Preventive and Therapeutic Agents for Alzheimer’s disease (AD). Curr. Med. Chem. 2011, 18, 3306–3313. [Google Scholar] [CrossRef]
- Chauhan, N.B. Anti-Amyloidogenic Effect of Allium sativum in Alzheimer’s Transgenic Model Tg2576. J. Herb. Pharmacother. 2003, 3, 95–107. [Google Scholar] [CrossRef]
- Mukherjee, D.; Banerjee, S. Learning and memory promoting effects of crude garlic extract. Indian J. Exp. Biol. 2013, 51, 1094–1100. [Google Scholar]
- Sorrenti, V.; Contarini, G.; Sut, S.; Dall’Acqua, S.; Confortin, F.; Pagetta, A.; Giusti, P.; Zusso, M. Curcumin Prevents Acute Neuroinflammation and Long-Term Memory Impairment Induced by Systemic Lipopolysaccharide in Mice. Front. Pharmacol. 2018, 9, 183. [Google Scholar] [CrossRef] [Green Version]
- Budzynska, B.; Boguszewska-Czubara, A.; Kruk-Slomka, M.; Skalicka-Woźniak, K.; Michalak, A.; Musik, I.; Biala, G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology 2015, 232, 931–942. [Google Scholar] [CrossRef]
- Ohba, T.; Yoshino, Y.; Ishisaka, M.; Abe, N.; Tsuruma, K.; Shimazawa, M.; Oyama, M.; Tabira, T.; Hara, H. Japanese Huperzia serrata extract and the constituent, huperzine A, ameliorate the scopolamine-induced cognitive impairment in mice. Biosci. Biotechnol. Biochem. 2015, 79, 1838–1844. [Google Scholar] [CrossRef]
- Rubio, J.; Dang, H.; Gong, M.; Liu, X.; Chen, S.-L.; Gonzales, G.F. Aqueous and hydroalcoholic extracts of Black Maca (Lepidium meyenii) improve scopolamine-induced memory impairment in mice. Food Chem. Toxicol. 2007, 45, 1882–1890. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Bhattacharya, A.; Kumar, A.; Ghosal, S. Antioxidant activity of Bacopa monniera in rat frontal cortex, stria-tum and hippocampus. Phytother. Res. 2000, 14, 174–179. [Google Scholar] [CrossRef]
- Chaudhari, K.S.; Tiwari, N.R.; Tiwari, R.R.; Sharma, R.S. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer’s disease. Ann. Neurosci. 2017, 24, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Shinomol, G.K.; Bharath, M.M.S. Muralidhara Neuromodulatory Propensity of Bacopa monnieri Leaf Extract Against 3-Nitropropionic Acid-Induced Oxidative Stress: In Vitro and In Vivo Evidences. Neurotox. Res. 2011, 22, 102–114. [Google Scholar] [CrossRef]
- Jyoti, A.; Sharma, D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology 2006, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Saoji, A.; Kumar, N.; Thawani, V.; Tiwari, M.; Thawani, M. Effect of Bacopa monnieri on Cognitive functions in Alzheimer’s disease patients. Int. J. Collab. Res. Intern. Med. Public Health 2011, 3, 285–293. [Google Scholar]
- Yassin Nemat, A.Z.; El-Shenawy, S.M.A.; Mahdy, K.A.; Gouda, N.A.M.; Marrie, A.E.-F.H.; Farrag, A.R.H.; Bassant, M.M. Ibrahim. Effect of Boswellia serrata on Alzheimer’s disease induced in rats. J. Arab Soc. Med. Res. 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Alama, B.; Haque, E. Anti-Alzheimer Antioxidant Activity of Celastrus paniculatus Seed. Iran J. Pharm. Sci. Winter 2011, 7, 49–56. [Google Scholar]
- Saxena, G.; Singh, S.P.; Pal, R.; Singh, S.; Pratap, R.; Nath, C. Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol. Biochem. Behav. 2007, 86, 797–805. [Google Scholar] [CrossRef]
- Nahata, A.; Patil, U.K.; Dixit, V.K. Effect of Evolvulus alsinoides Linn. on learning behavior and memory enhancement activity in rodents. Phytotherapy Res. 2009, 24, 486–493. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Cholinergic Basis of Memory-Strengthening Effect of Foeniculum vulgare Linn. J. Med. Food 2006, 9, 413–417. [Google Scholar] [CrossRef]
- Hager, K.; Kenklies, M.; McAfoose, J.; Engel, J.; Münch, G. α-Lipoic acid as a new treatment option for Alzheimer’s disease—A 48 months follow-up analysis. J. Neural Transm. Suppl. 2007, 72, 189–193. [Google Scholar] [CrossRef]
- Bickel, U.; Thomsen, T.; Fischer, J.; Weber, W.; Kewitz, H. Galanthamine: Pharmacokinetics, tissue distribution and cholinesterase inhibition in brain of mice. Neuropharmacology 1991, 30, 447–454. [Google Scholar] [CrossRef]
- Liu, X.; Hao, W.; Qin, Y.; Decker, Y.; Wang, X.; Burkart, M.; Schötz, K.; Menger, M.D.; Fassbender, K.; Liu, Y. Long-term treatment with Ginkgo biloba extract EGb 761 improves symptoms and pathology in a transgenic mouse model of Alzheimer’s disease. Brain Behav. Immun. 2015, 46, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-M.; Ao, M.-Z.; Li, W.; Yu, L.-J. Effect of Glabridin from Glycyrrhiza glabra on Learning and Memory in Mice. Planta Med. 2008, 74, 377–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Lee, Y.K.; Lee, B.J.; Nam, S.-Y.; Lee, S.I.; Kim, Y.H.; Kim, K.H.; Oh, K.-W.; Hong, J.T. Inhibitory effect of ethanol extract of Magnolia officinalis and 4-O-methylhonokiol on memory impairment and neuronal toxicity induced by beta-amyloid. Pharmacol. Biochem. Behav. 2010, 95, 31–40. [Google Scholar] [CrossRef]
- Soodi, M.; Naghdi, N.; Hajimehdipoor, H.; Choopani, S.; Sahraei, E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res. Pharm. Sci. 2014, 9, 107–114. [Google Scholar]
- Mahaman, Y.A.R.; Huang, F.; Wu, M.; Wang, Y.; Wei, Z.; Bao, J.; Salissou, M.T.M.; Ke, D.; Wang, Q.; Liu, R.; et al. Moringa oleifera Alleviates Homocysteine-Induced Alzheimer’s disease-Like Pathology and Cognitive Impairments. J. Alzheimer’s Dis. 2018, 63, 1141–1159. [Google Scholar] [CrossRef] [Green Version]
- Joshi, H.; Parle, M. Nardostachys jatamansi Improves Learning and Memory in Mice. J. Med. Food 2006, 9, 113–118. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jung, S.-W.; Kim, S.-Y.; Cho, I.-H.; Kim, H.-C.; Rhim, H.; Kim, M.; Nah, S.-Y. Panax ginseng as an adjuvant treatment for Alzheimer’s disease. J. Ginseng Res. 2018, 42, 401–411. [Google Scholar] [CrossRef]
- Uddin, S.; Al Mamun, A.; Hossain, S.; Ashaduzzaman, M.; Noor, A.A.; Hossain, S.; Uddin, J.; Sarker, J. Asaduzzaman Neuroprotective Effect of Phyllanthus acidus L. on Learning and Memory Impairment in Scopolamine-Induced Animal Model of Dementia and Oxidative Stress: Natural Wonder for Regulating the Development and Progression of Alzheimer’s disease. Adv. Alzheimer’s Dis. 2016, 05, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Sallam, A.; Mira, A.; Ashour, A.; Shimizu, K. Acetylcholine esterase inhibitors and melanin synthesis inhibitors from Salvia officinalis. Phytomedicine 2016, 23, 1005–1011. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. TLC-Bioautographic Evaluation of In Vitro Anti-tyrosinase and Anti-cholinesterase Potentials of Sandalwood Oil. Nat. Prod. Commun. 2013, 8, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Malini, S.; Bairy, K.L.; Rao, M.S.R. Effect of Tinospora cardifolia on learning and memory in normal and memory deficit rats. Indian J. Pharmacol. 2002, 34, 339–349. [Google Scholar]
- Patel, S.S.; Gupta, S.; Udayabanu, M. Urtica dioica modulates hippocampal insulin signaling and recognition memory deficit in streptozotocin induced diabetic mice. Metab. Brain Dis. 2016, 31, 601–611. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Kumar, A.; Ghosal, S. Effects of glycowithanolides from Withania somnifera on an animal model of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. Phytotherapy Res. 1995, 9, 110–113. [Google Scholar] [CrossRef]
- Mathew, M.; Subramaniam, S. In vitro evaluation of anti-Alzheimer effects of dry ginger (Zingiber officinale Roscoe) extract. Indian J. Exp. Biol. 2014, 52, 606–612. [Google Scholar]
- Acqua, S.D. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer’s disease. Bot. Targets Ther. 2013, 3, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Razay, G.; Wilcock, G.K. Galantamine in Alzheimer’s disease. Expert Rev. Neurother. 2008, 8, 9–17. [Google Scholar] [CrossRef]
- Maelicke, A.; Hoeffle-Maas, A.; Ludwig, J.; Maus, A.; Samochocki, M.; Jordis, U.; Koepke, A.K.E. Memogain is a Galantamine Pro-drug having Dramatically Reduced Adverse Effects and Enhanced Efficacy. J. Mol. Neurosci. 2009, 40, 135–137. [Google Scholar] [CrossRef]
- Nillert, N.; Pannangrong, W.; Welbat, J.U.; Chaijaroonkhanarak, W.; Sripanidkulchai, K.; Sripanidkulchai, B. Neuroprotective Effects of Aged Garlic Extract on Cognitive Dysfunction and Neuroinflammation Induced by β-Amyloid in Rats. Nutrients 2017, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Rebai, O.; Belkhir, M.; Sanchez-Gomez, M.V.; Matute, C.; Fattouch, S.; Amri, M. Differential Molecular Targets for Neuroprotective Effect of Chlorogenic Acid and its Related Compounds Against Glutamate Induced Excitotoxicity and Oxidative Stress in Rat Cortical Neurons. Neurochem. Res. 2017, 42, 3559–3572. [Google Scholar] [CrossRef]
- Ali, Y.O.; Bradley, G.; Lu, H.-C. Screening with an NMNAT2-MSD platform identifies small molecules that modulate NMNAT2 levels in cortical neurons. Sci. Rep. 2017, 7, 43846. [Google Scholar] [CrossRef] [PubMed]
- Thaipisuttikul, P.; Galvin, J.E. Use of medical foods and nutritional approaches in the treatment of Alzheimer’s disease. Clin. Pract. 2012, 9, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frydman-Marom, A.; Levin, A.; Farfara, R.; Benromano, T.; Scherzer-Attali, R.; Peled, S.; Vassar, R.; Segal, D.; Gazit, E.; Frenkel, D.; et al. Orally Administrated Cinnamon Extract Reduces β-Amyloid Oligomerization and Corrects Cognitive Impairment in Alzheimer’s disease Animal Models. PLoS ONE 2011, 6, e16564. [Google Scholar] [CrossRef] [Green Version]
- Rajakrishnan, V.; Viswanathan, P.; Rajasekharan, K.N.; Menon, V.P. Neuroprotective role of curcumin from Curcuma longa on ethanol-induced brain damage. Phytother. Res. 1999, 13, 571–574. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449–4460. [Google Scholar] [CrossRef] [Green Version]
- Cole, G.M.; Ma, Q.-L.; Frautschy, S.A. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s disease Mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Wang, M.; Li, W.; Matsumoto, K.; Tang, Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007, 80, 1373–1381. [Google Scholar] [CrossRef]
- Williams, P.; Sorribas, A.; Howes, M.-J.R. Natural products as a source of Alzheimer’s drug leads. Nat. Prod. Rep. 2010, 28, 48–77. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Shi, J.S. SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 75, 385–395. [Google Scholar] [CrossRef]
- Moreno, L.C.G.E.I.; Puerta, E.; Suárez-Santiago, J.E.; Santos-Magalhães, N.S.; Ramirez, M.J.; Irache, J.M. Effect of the oral administration of nanoencapsulated quercetin on a mouse model of Alzheimer’s disease. Int. J. Pharm. 2017, 517, 50–57. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Teles, R.B.; Diniz, T.C.; Costa Pinto, T.C.; de Oliveira Junior, R.G.; Gama, E.S.M.; de Lavor, E.M.; Fernandes, A.W.C.; de Oliveira, A.P.; de Almeida Ribeiro, F.P.R.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxid. Med. Cell. Longev. 2018, 2018, 7043213. [Google Scholar] [CrossRef]
- Kumar, A.; Nisha, C.M.; Silakari, C.; Sharma, I.; Anusha, K.; Gupta, N.; Nair, P.; Tripathi, T.; Kumar, A. Current and novel therapeutic molecules and targets in Alzheimer’s disease. J. Formos. Med. Assoc. 2016, 115, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, L.; Tabet, N. Slowing the progression of Alzheimer’s disease; what works? Ageing Res. Rev. 2015, 23, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Gauthier, S. Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer’s disease. Drugs Aging. 2001, 8, 853–862. [Google Scholar] [CrossRef]
- Crismon, M.L. Tacrine: First Drug Approved for Alzheimer’s disease. Ann. Pharmacother. 1994, 28, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Qizilbash, N.; Birks, J.; Arrieta, J.L.; Lewington, S.; Szeto, S. Tacrine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2000, 2, CD000202. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, T.; Uddin, S.; Begum, M.M.; Thangapandiyan, S.; Rahman, S.; Aleya, L.; Mathew, B.; Ahmed, M.; Barreto, G.E.; Ashraf, G. Cholinesterase Inhibitors for Alzheimer’s disease: Multitargeting Strategy Based on Anti-Alzheimer’s Drugs Repositioning. Curr. Pharm. Des. 2019, 25, 3519–3535. [Google Scholar] [CrossRef]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007, 3, 303–333. [Google Scholar] [PubMed]
- Kumar, A.; Sharma, S. Donepezil. In StatPearl; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513257/ (accessed on 8 December 2020).
- Dooley, M.; Lamb, H.M. Donepezil: A review of its use in Alzheimer’s disease. Drugs Aging 2000, 16, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s disease: Getting On and Staying On. Curr. Ther. Res. 2003, 64, 216–235. [Google Scholar] [CrossRef] [Green Version]
- Annicchiarico, R.; Federici, A.; Pettenati, C.; Caltagirone, C. Rivastigmine in Alzheimer’s disease: Cognitive function and quali-ty of life. Ther. Clin. Risk Manag. 2007, 3, 1113–1123. [Google Scholar]
- Müller, T. Rivastigmine in the treatment of patients with Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2007, 3, 211–218. [Google Scholar] [CrossRef]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Birks, J.; Evans, J.G.; Iakovidou, V.; Tsolaki, M. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. 2009, 4, CD001191. [Google Scholar] [CrossRef]
- Scott, L.J.; Goa, K.L. Galantamine. Drugs 2000, 60, 1095–1122. [Google Scholar] [CrossRef] [PubMed]
- Prvulovic, D.; Hampel, H.; Pantel, J. Galantamine for Alzheimer’s disease. Expert Opin. Drug Metab. Toxicol. 2010, 6, 345–354. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Pharmacological aspects of galantamine for the treatment of Alzheimer’s disease. EXCLI J. 2017, 16, 35–39. [Google Scholar] [CrossRef]
- Wahba, S.M.; Darwish, A.S.; Kamal, S.M. Ceria-containing uncoated and coated hydroxyapatite-based galantamine nanocomposites for formidable treatment of Alzheimer’s disease in ovariectomized albino-rat model. Mater. Sci. Eng. C 2016, 65, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s disease. Front. Neurosci. 2019, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-J.; Lin, C.-H.; Lane, H.-Y.; Tsai, G.E. NMDA Neurotransmission Dysfunction in Behavioral and Psychological Symptoms of Alzheimer’s disease. Curr. Neuropharmacol. 2012, 10, 272–285. [Google Scholar] [CrossRef] [Green Version]
- Companys-Alemany, J.; Turcu, A.L.; Bellver-Sanchis, A.; Loza, M.I.; Brea, J.M.; Canudas, A.M.; Leiva, R.; Vázquez, S.; Pallàs, M.; Griñán-Ferré, C. A Novel NMDA Receptor Antagonist Protects against Cognitive Decline Presented by Senescent Mice. Pharmaceutics 2020, 12, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the Treatment of Dementia: A Review on its Current and Future Applications. J. Alzheimer’s Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.H.; Mir, M.; Ngowi, E.E.; Zafar, U.; Khakwani, M.M.A.K.; Khattak, S.; Zhai, Y.-K.; Jiang, E.-S.; Zheng, M.; Duan, S.-F.; et al. Nanomedicine: A Promising Way to Manage Alzheimer’s disease. Front. Bioeng. Biotechnol. 2021, 9, 630055. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, T.T.D.; Vo, T.K.; Tran, N.-M.; Nguyen, M.K.; Van Vo, T.; Van Vo, G. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021, 143, 112117. [Google Scholar] [CrossRef] [PubMed]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; Le Droumaguet, B.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordoñez-Gutierrez, L.; et al. Antibody-functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s disease-like transgenic mouse model. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 609–618. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C. Memantine loaded PLGA PEGylated nanoparti-cles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.G.; Cha, M.-Y.; Kim, J.-I.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.-J.; Moon, M. Vitamin D-binding protein-loaded PLGA nanoparticles suppress Alzheimer’s disease-related pathology in 5XFAD mice. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Vilella, A.; Belletti, D.; Sauer, A.K.; Hagmeyer, S.; Sarowar, T.; Masoni, M.; Stasiak, N.; Mulvihill, J.J.; Ruozi, B.; Forni, F.; et al. Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J. Trace Elements Med. Biol. 2018, 49, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Alobaid, B.N.M.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2020, 61, 102176. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal Piperine-Loaded Chitosan Nanoparticles as Brain-Targeted Therapy in Alzheimer’s disease: Optimization, Biological Efficacy, and Potential Toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Kim, H.-S.; Cho, E.-K.; Kwon, B.-Y.; Phark, S.; Hwang, K.-W.; Sul, D. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008, 46, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Wood, T.; Chen, C.C.; Corry, K.; Snyder, J.M.; Juul, S.E. Curcumin-loaded polymeric nanoparticles for neuroprotection in neonatal rats with hypoxic-ischemic encephalopathy. Nano Res. 2018, 11, 5670–5688. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C.-L. In Vitro and In Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly(lactic-co-glycolic acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Kanwar, A.; Vegh, C.; Marginean, A.; Elliott, A.; Guilbeault, N. Ubisol-Q 10 (a nanomicellar water-soluble formulation of CoQ 10) treatment inhibits Alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (TgAPEswe, PSEN1dE9) model of Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 221–236. [Google Scholar] [CrossRef]
- Hagl, S.; Kocher, A.; Schiborr, C.; Kolesova, N.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in neuronal PC12 cells and brains of NMRI mice—Impact on bioavailability. Neurochem. Int. 2015, 89, 234–242. [Google Scholar] [CrossRef]
- Mirzaie, Z.; Ansari, M.; Kordestani, S.S.; Rezaei, M.H.; Mozafari, M. Preparation and characterization of curcumin-loaded polymeric nanomicelles to interference with amyloidogenesis through glycation method. Biotechnol. Appl. Biochem. 2017, 66, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Riaz, M.K.; Xie, Y.; Zhang, X.; Liu, Q.; Chen, H.; Bian, Z.; Chen, X.; Lu, A.; Yang, Z. Review of Current Strategies for Delivering Alzheimer’s disease Drugs across the Blood-Brain Barrier. Int. J. Mol. Sci. 2019, 20, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliev, G.; Ashraf, G.M.; Tarasov, V.V.; Chubarev, V.N.; Leszek, J.; Gasiorowski, K.; Makhmutova, A.; Baeesa, S.S.; Avila-Rodríguez, M.; Ustyugov, A.A.; et al. Alzheimer’s disease—Future Therapy Based on Dendrimers. Curr. Neuropharmacol. 2019, 17, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin Dutta, A.; Borah, A. Lactoferrin coupled lower generation PAMAM den-drimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer’s disease in mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Aso, E.; Martinsson, I.; Appelhans, D.; Effenberg, C.; Benseny Cases, N.; Cladera, J. Poly (propylene imine) dendrimers with histidine-maltose shell as novel type of nanoparticles for synapse and memory protection. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Serafín, V.; Razzino, C.A.; Gamella, M.; Pedrero, M.; Povedano, E.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; et al. Disposable immunoplatforms for the simultaneous determination of biomarkers for neurodegenerative disorders using poly(amidoamine) dendrimer/gold nanoparticle nanocomposite. Anal. Bioanal. Chem. 2020, 413, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Naki, T. Design and Efficacy of Nanogels Formulations for Intranasal Administration. Molecules 2018, 23, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picone, P.; Sabatino, M.A.; Ditta, L.A.; Amato, A.; Biagio, P.L.S.; Mulè, F.; Giacomazza, D.; Dispenza, C.; Di Carlo, M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 2018, 270, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines 2018, 5, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arduino, I.; Iacobazzi, R.M.; Riganti, C.; Lopedota, A.A.; Perrone, M.G.; Lopalco, A.; Cutrignelli, A.; Cantore, M.; Laquintana, V.; Franco, M.; et al. Induced expression of P-gp and BCRP transporters on brain endothelial cells using transferrin functionalized nanostructured lipid carriers: A first step of a potential strategy for the treatment of Alzheimer’s disease. Int. J. Pharm. 2020, 591, 120011. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Rosini, M.; Racchi, M.; Lanni, C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017, 124, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Malvajerd, S.S.; Izadi, Z.; Azadi, A.; Kurd, M.; Derakhshankhah, H.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Neuroprotective Potential of Curcumin-Loaded Nanostructured Lipid Carrier in an Animal Model of Alzheimer’s disease: Behavioral and Biochemical Evidence. J. Alzheimer’s Dis. 2019, 69, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S. Targeted drug delivery across the blood brain barrier in Alzheimer’s disease. Curr. Pharm. Des. 2013, 19, 6635–6646. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Layek, B.; Singh, J. Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood–Brain Barrier for Effective Treatment of Alzheimer’s disease. Mol. Pharm. 2020, 18, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, T.S.; Mothilal, M. Development of rivastigmine loaded self-assembled nanostructures of nonionic surfactants for brain delivery. Int. J. Appl. Pharm. 2021, 13, 205–215. [Google Scholar] [CrossRef]
- Ansari, M.; Eslami, H. Preparation and study of the inhibitory effect of nano-niosomes containing essential oil from artemisia absinthium on amyloid fibril formation. Nanomed. J. 2020, 7, 243–250. [Google Scholar] [CrossRef]
- Kulkarni, P.; Rawtani, D.; Barot, T. Design, development and in-vitro/in-vivo evaluation of intranasally delivered Rivastigmine and N-Acetyl Cysteine loaded bifunctional niosomes for applications in combinative treatment of Alzheimer’s disease. Eur. J. Pharm. Biopharm. 2021, 163, 1–15. [Google Scholar] [CrossRef]
- Kaur, A.; Nigam, K.; Srivastava, S.; Tyagi, A.; Dang, S. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef]
- Patil, R.P.; Pawara, D.D.; Gudewar, C.S.; Tekade, A.R. Nanostructured cubosomes in an in situ nasal gel system: An alternative approach for the controlled delivery of donepezil HCl to brain. J. Liposome Res. 2018, 29, 264–273. [Google Scholar] [CrossRef]
- Sintov, A.C. AmyloLipid Nanovesicles: A self-assembled lipid-modified starch hybrid system constructed for direct nose-to-brain delivery of curcumin. Int. J. Pharm. 2020, 588, 119725. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, S.; Rajeshkumar, S. Gold nanoparticles in diagnosis and treatment of Alzheimer’s disease. In Nanobiotechnology in Neurodegenerative Diseases; Mahendra, R., Alka, Y., Eds.; Springer: Cham, Switzerland, 2019; pp. 289–306. [Google Scholar] [CrossRef]
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A. Impact of gold nanoparticles on amyloid β-induced Alzheimer’s disease in a rat animal model: Involvement of STIM Proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G. Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Mol. Neurobiol. 2020, 57, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Dao, P.; Chen, H.; Yan, L.; Li, Y.L.; Zhang, W.; Li, L.; Du, Z.; Dong, C.-Z.; Meunier, B. Ultrasmall superparamagnetic iron oxide nanoparticles-bound NIR dyes: Novel theranostic agents for Alzheimer’s disease. Dyes Pigment. 2020, 173, 107968. [Google Scholar] [CrossRef]
- Luo, S.; Ma, C.; Zhu, M.-Q.; Ju, W.-N.; Yang, Y.; Wang, X. Application of Iron Oxide Nanoparticles in the Diagnosis and Treatment of Neurodegenerative Diseases with Emphasis on Alzheimer’s disease. Front. Cell. Neurosci. 2020, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Lockman, P.; Atwood, C.S.; Hsu, C.-H.; Gupte, A.; Allen, D.D.; Mumper, R.J. Novel d-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer’s and other CNS diseases. Eur. J. Pharm. Biopharm. 2005, 59, 263–272. [Google Scholar] [CrossRef]
- Liu, G.; Men, P.; Kudo, W.; Perry, G.; Smith, M.A. Nanoparticle–chelator conjugates as inhibitors of amyloid-β aggregation and neurotoxicity: A novel therapeutic approach for Alzheimer’s disease. Neurosci. Lett. 2009, 455, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Men, P.; Harris, P.L.; Rolston, R.K.; Perry, G.; Smith, M.A. Nanoparticle iron chelators: A new therapeutic approach in Alzheimer’s disease and other neurologic disorders associated with trace metal imbalance. Neurosci. Lett. 2006, 406, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.R.; Lepene, B.S.; Thatcher, C.D.; Long, T.E. Synthesis and Characterization of Poly(ethylene glycol)−Glutathione Conjugate Self-Assembled Nanoparticles for Antioxidant Delivery. Biomacromolecules 2008, 10, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ji, C.; Xu, H.; Li, X.; Ding, H.; Ye, M.; Zhu, Z.; Ding, D.; Jiang, X.; Ding, X.; et al. Resveratrol-loaded polymeric micelles protect cells from Aβ-induced oxidative stress. Int. J. Pharm. 2009, 375, 89–96. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.S.; Paramakrishnan, N.; Suresh, B. Targeted delivery of tacrine into the brain with polysorbate 80-coated poly(n-butylcyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2008, 70, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Fasae, K.D.; Abolaji, A.O.; Faloye, T.R.; Odunsi, A.Y.; Oyetayo, B.O.; Enya, J.I.; Rotimi, J.A.; Akinyemi, R.O.; Whitworth, A.J.; Aschner, M. Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer’s disease: Limitations, and current and future perspectives. J. Trace Elem. Med. Biol. 2021, 67, 126779. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Choi, H.J.; Jung, D.C.; Lee, J.-H.; Cheon, J. Nanoparticle assisted magnetic resonance imaging of the early reversible stages of amyloid β self-assembly. Chem. Commun. 2008, 19, 2197–2199. [Google Scholar] [CrossRef]
- Schaffazick, S.; Pohlmann, A.; de Cordova, C.; Creczynski-Pasa, T.; Guterres, S. Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int. J. Pharm. 2005, 289, 209–213. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.K.; Kumar, K.S.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer’s drug tacrine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef]
- Hã¤Rtig, W.; Kacza, J.; Paulke, B.-R.; Grosche, J.; Bauer, U.; Hoffmann, A.; Elsinghorst, P.W.; Gütschow, M.; Gütschow, M. In vivo labelling of hippocampal β-amyloid in triple-transgenic mice with a fluorescent acetylcholinesterase inhibitor released from nanoparticles. Eur. J. Neurosci. 2009, 31, 99–109. [Google Scholar] [CrossRef]
- Skaat, H.; Margel, S. Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-β fibrils detection and removal by a magnetic field. Biochem. Biophys. Res. Commun. 2009, 386, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.S.; Rubinstein, I.; Önyüksel, H. PEGylated phospholipid nanomicelles interact with β-amyloid(1–42) and mitigate its β-sheet formation, aggregation and neurotoxicity in vitro. Peptides 2006, 27, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Manek, E.; Petroianu, G.A. Chitosan-based nanoparticles in Alzheimer’s disease: Messenger or message? Neural Regen. Res. 2021, 16, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Boridy, S.; Takahashi, H.; Akiyoshi, K.; Maysinger, D. The binding of pullulan modified cholesteryl nanogels to Aβ oligomers and their suppression of cytotoxicity. Biomaterials 2009, 30, 5583–5591. [Google Scholar] [CrossRef] [PubMed]
- Agyare, E.K.; Curran, G.L.; Ramakrishnan, M.; Yu, C.C.; Poduslo, J.F.; Kandimalla, K.K. Development of a Smart Nano-vehicle to Target Cerebrovascular Amyloid Deposits and Brain Parenchymal Plaques Observed in Alzheimer’s disease and Cerebral Amyloid Angiopathy. Pharm. Res. 2008, 25, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, Z.; Cheng, P.; He, Z.; Cheng, Z.; Peng, J.; Wang, H.; Wang, C.; Yang, Y.; Hu, Z. Antibody-Mimetic Peptoid Nanosheet for Label-Free Serum-Based Diagnosis of Alzheimer’s disease. Adv. Mater. 2017, 29, 1700057. [Google Scholar] [CrossRef]
- Araya, E.; Olmedo, I.; Bastus, N.G.; Guerrero, S.; Puntes, V.F.; Giralt, E.; Kogan, M.J. Gold Nanoparticles and Microwave Irradiation Inhibit Beta-Amyloid Amyloidogenesis. Nanoscale Res. Lett. 2008, 3, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.A.; Henry, J.E.; Good, T.A. Attenuation of β-amyloid-induced toxicity by sialic-acid-conjugated dendrimers: Role of sialic acid attachment. Brain Res. 2007, 1161, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Dai, F.; Fan, Z.; Ma, G.; Tang, Q.; Zhang, X. Nanotheranostics: Congo Red/Rutin-MNPs with Enhanced Magnetic Resonance Imaging and H2O2-Responsive Therapy of Alzheimer’s disease in APPswe/PS1dE9 Transgenic Mice. Adv. Mater. 2015, 27, 5499–5505. [Google Scholar] [CrossRef]
- Klajnert, B.; Cladera, J.; Bryszewska, M. Molecular Interactions of Dendrimers with Amyloid Peptides: pH Dependence. Biomacromolecules 2006, 7, 2186–2191. [Google Scholar] [CrossRef]
- Mondal, S.; Chowdhury, S.R.; Shah, M.; Kumar, V.; Kumar, S.; Iyer, P.K. Nanoparticle Assisted Regulation of Nucleation Pathway of Amyloid Tetramer and Inhibition of Their Fibrillation Kinetics. ACS Appl. Bio. Mater. 2019, 2, 2137–2142. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Lindman, S.; Minogue, A.M.; Thulin, E.; Walsh, D.M.; Dawson, K.A.; Linse, S. Inhibition of Amyloid β Protein Fibrillation by Polymeric Nanoparticles. J. Am. Chem. Soc. 2008, 130, 15437–15443. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Kumar, V.; Chowdhury, S.R.; Shah, M.; Gaur, A.; Kumar, S.; Iyer, P.K. Template-Mediated Detoxification of Low-Molecular-Weight Amyloid Oligomers and Regulation of Their Nucleation Pathway. ACS Appl. Bio. Mater. 2019, 2, 5306–5312. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Dawson, K.A.; Linse, S. Dual Effect of Amino Modified Polystyrene Nanoparticles on Amyloid β Protein Fibrillation. ACS Chem. Neurosci. 2010, 1, 279–287. [Google Scholar] [CrossRef]
- Mourtas, S.; Canovi, M.; Zona, C.; Aurilia, D.; Niarakis, A.; La Ferla, B.; Salmona, M.; Nicotra, F.; Gobbi, M.; Antimisiaris, S. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials 2011, 32, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Bolade, O.P.; Williams, A.B.; Benson, N.U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manag. 2019, 13, 100279. [Google Scholar] [CrossRef]
- Faisal, S.; Jan, H.; Shah, S.A.; Shah, S.; Khan, A.; Akbar, M.T.; Rizwan, M.; Jan, F.; Wajidullah; Akhtar, N.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, V.; Richardson, R.M. Gene Therapy for Neurodegenerative Diseases. Neurotherapeutics 2018, 16, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soofiyani, S.R.; Baradaran, B.; Lotfipour, F.; Kazemi, T.; Mohammadnejad, L. Gene Therapy, Early Promises, Subsequent Problems, and Recent Breakthroughs. Adv. Pharm. Bull. 2013, 3, 249–255. [Google Scholar] [CrossRef]
- Jadhav, S.; Avila, J.; Schöll, M.; Kovacs, G.G.; Kövari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; De Silva, R.; et al. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef]
- Pardridge, W.M. Peptide Drug Delivery to the Brain; Raven Press: New York, NY, USA, 1991; pp. 1–357. [Google Scholar]
- Pardridge, W.M. Drug Delivery to the Brain. J. Cereb. Blood Flow Metab. 1997, 17, 713–731. [Google Scholar] [CrossRef] [PubMed]
- Juliano, R.L. The delivery of therapeutic oligonucleotides. Nucleic. Acids Res. 2016, 44, 6518. [Google Scholar] [CrossRef] [PubMed]
- Khorkova, O.; Wahlestedt, C. Oligonucleotide therapies for disorders of the nervous system. Nat. Biotechnol. 2017, 35, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Pulst, S.M. Spinocerebellar Ataxia Type 2. Polyglutamine Disord. 2018, 1049, 175–195. [Google Scholar] [CrossRef]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef]
- Becker, L.A.; Huang, B.; Bieri, G.; Ma, R.; Knowles, D.A.; Jafar-Nejad, P.; Messing, J.; Kim, H.J.; Soriano, A.; Auburger, G.; et al. Therapeutic reduction of ataxin-2 extends lifespan and reduces pathology in TDP-43 mice. Nature 2017, 544, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Scoles, D.R.; Meera, P.; Schneider, M.D.; Paul, S.; Dansithong, W.; Figueroa, K.P.; Hung, G.; Rigo, F.; Bennett, G.H.F.R.C.F.; Otis, P.M.T.S.; et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature 2017, 544, 362–366. [Google Scholar] [CrossRef]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy. Neurology 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Investigational Drug/Nutraceutical (Category) | Mechanism of Action | Sponsor (Clinical Trial End Date) |
|---|---|---|
| Aducanumab (monoclonal antibody (MA)) | Remove amyloid plaque | Biogen (April 2022) |
| Crenezumab (MA) | Roche/Genentech (July 2021) | |
| Gantenerumab (MA) | Roche (November 2019) | |
| Solanezumab (MA) | Eli Lilly (July 2022) | |
| CNP520 (amyloid vaccine) | Reduces amyloid formation by inhibiting APP site cleavage | Axsome therapeutics (July 2024) |
| Methylphenidate (neurotransmitter-based) | Improves clinical symptoms by inhibiting dopamine re-uptake | Johns Hopkins (August 2020) |
| ABBV-8E12 (MA) | Removes tau protein | Abbvie (June 2021) |
| Cilostazol (vasodilator) | PDE3 antagonist | National Cerebral & Cardiovascular Center, Japan (December 2020) |
| Telmisartan (angiotensin receptor blocker) | Improve vascular functioning | Sunnybrook Health Sciences Centre (March 2021) |
| Deferipirone (iron chelator) | Reduces reactive oxygen species (ROS) that can harm neurons, making it neuroprotective | Neuroscience trials, Australia (December 2021) |
| Dronabinol (CB1 and CB2 endocannabinoids partial agonist) | Improve agitation (neuropsychiatric symptoms in AD) | John Hopkins University (December 2020) |
| Icosapent ethyl (purified form of Omega 3 fatty acid EPA (Omega 3 FA) | Neuroprotective, affords protection from disease pathology | University of Wisconsin (November 2021) |
| Grape seed extract (nutraceutical) | Anti-oligomerization agent | Mount Sinai AD Research Center (September 2020) |
| Botanical Name (Common Name) | Family | Plant Part/Extract Used | Phytoconstituent/s for Treatment of AD | Model/Method | Probable Mechanism of Action | Reference(s) |
|---|---|---|---|---|---|---|
| Acorus calamus (Sweet flag) | Araceae | Roots; hydro-ethanolic (70:30) extract; methanolic Extract [70] | α and β asarone | Lipopolysaccharide-induced neuroinflammation in a model of rat [71]; Ellman method [70] | Reduces oxidative stress and has anti-inflammatory properties; inhibition of acetylcholinesterase (AchE) | [72,73] |
| Allium sativum (Garlic) | Alliaceae | Bulbs | Di-allyl-disulfide and s-allyl cysteine | Scopolamine-induced amnesia; transgenic mouse model Tg2576 | Anti-AchE activity, neuroprotective, antioxidant, hypocholesterolemic, reduces Aβ biomarker | [74,75,76] |
| Angelica archangelica (Wild Celery) | Apiaceae | Methanolic extract of fruits | Furanocoumarins like imperatorin, xanthotoxin | Scopolamine-induced mice model [77] | Scopolamine-induced memory impairment is improved; the inhibition of AChE | [78] |
| Bacopa monniera (Brahmi) | Scrophulariaceae | Hydroethanolic extract of leaves and stems; methanolic extract | Bitulinic acid, stigmasterol bacosides A & B, sapogenins & apigenin [79,80] | In vitro oxidative stress caused by aluminum in the hippocampus; oxidative damage caused by 3-nitropropionic acid | Inhibition of AchE improves cognition; enhances free radical scavenging mechanism; neuroprotective. | [81,82,83,84,85] |
| Boswellia serrata: (Salai guggul) | Burseraceae | Aqueous extract of Gum resin | Boswellic acids | Neurotoxicity caused by aluminum chloride in a rat model | Increase in acetylcholine levels in the brain | [86] |
| Celastrus paniculatus (Black oil plant) | Celastraceae | Methanolic extract of Seed | Wifornine F, paniculatine A and B | AchE assay; DPPH antioxidant assay | Inhibition of AchE and DPPH assay signifies scavenging of free radicals | [87] |
| Centella asiatica (Gotu kola) | Apiaceae | Aqueous extract of whole aerial plant | Asiatic and madecassic acid | STZ-induced oxidative stress and rat model of cognitive impairment | Lower level of Aβ biomarker which improves cognition and enhances antioxidant defense mechanisms | [69] |
| Commiphora whighitti (Guggul) | Burseraceae | Ethyl acetate extract of resin | Guggulipid, guggulsterone | STZ-induced memory deficits model | Anti-AchE, anti-oxidant, and hypolipidemic activities | [88] |
| Convolvulus pluricaulis (Shankhpushpi) | Convolvulaceae | Aqueous extract of roots | Shankhapushpine | Aluminum-induced neurotoxicity model; tau-induced neurotoxicity in the Drosophila fly model | Neuroprotection; a decrease in τ protein levels, i.e., a reduction in τ-induced oxidative stress is beneficial | [71] |
| Curcuma longa (Turmeric) | Zingiberaceae | Methanolic extract of rhizome | Curcumin | Transgenic mice (chronic model) LPS-induced neuroinflammation model | Minimize interleukin-1 β levels, decreased Janus Kinase mediate transcription, and prevented Aβ aggregation Reduction in neuroinflammation due to anti-inflammatory properties | [70,77] |
| Evolvulus alsinoides (Nela kuriji) | Convolvulaceae | Ethyl acetate extract of aerial part | Scopoletin | Shuttle box avoidance and step down paradigm and model | Nootropic activity | [89] |
| Foeniculum vulgare (Fennel) | Apiaceae | Methanolic extract from fruits | Quercetin, rosmarinic acid, 3-caffeoylquinic acid, gallic acid, and kaempferol | Scopolamine memory deficit model | Increase in AchE inhibition | [90] |
| Galanthus nivalis (Snowdrop) | Amaryllidaceae | Aqueous extract of bulbs | Galanthamine | Scopolamine-induced model [91] | AchE inhibitor activity | [92] |
| Ginkgo biloba (Maiden hair tree) | Ginkgoaceae | Leaves | Ginkgoflavonglycosides & isorhamnetin | Amyloid precursor protein-transgenic mouse model | Neuroprotection by lowering APP levels | [93] |
| Glycyrrhiza glabra (Licorice) | Fabaceae | Acetone extract of root | Glabridin | Scopolamine-induced model | Reduction in the brain cholinesterase activity | [94] |
| Huperzia serrata (Toothed clubmoss) | Lycopodiaceae | Hydroethanol extract of aerial parts | Huperzine A | In albino male mice, there is memory loss | AchE inhibition, neuroprotection | [79] |
| Lipidium Meyenii Walp (Peruvian ginseng) | Brassicaceae | Aqueous and hydroalcoholic extracts of hypocotyls | (1R,3S)-1-methyltetrahydro-beta-carboline-3-carboxylic acid | Scopolamine model | Inhibition of AchE activity | [80] |
| Magnolia officinalis (Houpu magnolia) | Magnoliaceae | Ethanolic extract of bark | 4-O-methylhonokiol | Aβ mouse model for neuronal toxicity | Neuroprotection | [95] |
| Melissa officinalis (Common balm) | Lamiaceae | Ethanolic extract of leaves | Rosmarinic acid | Scopolamine-induced rat model | Inhibition of AChE activity | [96] |
| Moringa olifera (Drumstick tree) | Moringaceace | Hydro-methanolic leaf extract (20:80) | - | Hyperhomocysteinemia (HHcy) induced AD | Improved the homocysteine-induced oxidative stress | [97] |
| Nardostachys jatamansi (Spikenard) | Valerianaceae | Ethanolic extract of roots | Nardostachysin, jatamansin, jatamols A and B | Diazepam and scopolamine induced amnesia in mice models | Increases cholinergic transmission, as well as neuroprotection and anti-oxidant activity | [98] |
| Panax Ginseng (Ginseng) | Araliaceae | Alcoholic extract of roots | Gintonin, ginsenosides | Mice model for Aβ induced neurotoxicity | Activates the cholinergic system while inhibiting β and γ-secretase activity; reduced synaptophysin and choline acetyltransferase activity are restored to normal levels | [99] |
| Phyllanthus acidus (Malay gooseberry) | Phyllanthaceae | Methanolic extract | - | Scopolamine-induced animal model of dementia and oxidative stress and elevated plus maze test | Decreasing lipid peroxidation and acetylcholinesterase activity | [100] |
| Rosmarinus officinalis (Rosemary) | Lamiaceae | Hydroethanolic extract from leaves | Rosmarinic acid | Scopolamine-induced rat model | Decrease of AchE activity in the brain | [101] |
| Salvia officinalis (Sage) | Lamiaceae | Methanolic extract of dry aerial parts | Carnosol, methoxyrosmanol, epirosmanol | Ellman method | AChE inhibition is dose-dependent | [102] |
| Santalum album (Indian sandalwood) | Santalaceae | Oil | Alpha-santalol | TLC-bioautographic assessment and colorimetric method | Tyrosinase and cholinesterase inhibition | [103] |
| Tinospora cordifolia (Giloya) | Menispermaceae | Aqueous extract of the whole plant | Choline | In a rat model, cyclosporine caused a memory loss | Immunostimulation as well as enhancing the synthesis of the neurotransmitter acetylcholine | [104] |
| Urtica dioica L. (Stinging nettle) | Urticaceae | Hydromethanolic extract of leaves | Kaempferol, isorhamnetin, chlorogenic acid, scopoletin | STZ-induced diabetic mice model | Modulates glucose homeostasis in the hippocampus | [105] |
| Withania somnifera (Ashwagandha) | Solanaceae | Aqueous and methanolic root extract | Withanamides | Amyloid peptide induced memory deficit | Cognition-enhancing and memory-improving effects | [106] |
| Zingiber officinalis (Ginger) | Zingiberaceae | Methanolic extract of rhizomes | Flavonoid & polyphenol | DPPH method & Ellman method | Inhibition of acetylcholinesterase (AchE) | [107] |
| (a) | |||||
| S. No | Nanoparticle | Application | |||
| 1 | MPB-PE & PDP-PE NPs [194] | Copper Aβ aggregates are solubilized when conjugated with D-penicillamine | |||
| 2 | (CMC)-Nano-N2PY [195] | Accompanied by the pyridinone MAEHP, which is capable of dissolving and solubilizing iron aggregates. | |||
| 3 | Nanoparticles coated with polysorbate 80 (CNPS and ICNPS) [196] | MHP: iron removal, ApoE, and ApoA-I binding | |||
| (b) | |||||
| S. No | Nanoparticle | Application | |||
| 4 | Nanoparticles (PLGA) [197] | CQ10 (coenzyme)-enriched NP | |||
| 5 | A PEG-coated poly caprolactone core [198] | Resveratrol-loaded nanoparticles diminish Aβ associated toxicity in cellular systems | |||
| 6 | PEG-GSH conjugate NP [199] | GSH NP: reduces oxidative stress in cells | |||
| 7 | PLGA NP [200] | Encapsulated superoxidase dismutase NP: protection against H2O2-induced oxidative stress | |||
| 8 | Solid lipid NP [201] | Aβ42 prevents the production of intracellular ROS by ferulic acid-loaded NP | |||
| (c) | |||||
| S. No. | Nanoparticle | Application | |||
| 1 | Chitosan NP, PBCA NP [202] | Tacrine-loaded NP | |||
| 2 | PBCA NP with polysorbate 80 coating [203] | Rivastagmine-loaded NP | |||
| 3 | Composite polystyrene/butylcyanoacrylate [204] | Aβ carrier of anti-acetylcholine esterase inhibitor PE154-loaded | |||
| (d) | |||||
| Amyloid Cascade Hypothesis | Amyloid Cascade Hypothesis | ||||
| S. No. | Nanoparticle | Application | S. No. | Nanoparticle | Application |
| 1 | Polystyrene core PBCA shell [205] | Thioflavin T-loaded NP | 9 | DSPE-PEG2000 nanomicelle [206] | Inhibit Aβ aggregation, diminish beta sheet formation |
| 2 | Chitosan NP [207] | Aβ rich fragment: improves humoral immunity and decreases Aβ burden | 10 | Pullulan nanogel [208] | NP regulates Aβ aggregate formation |
| 3 | Chitosan polymeric NPs [209] | Anti-amyloid antibody-loaded | 11 | KLVFF functionalized nanodevice: KLVFFGG]4 peptide nanosheet [210] | Potentiates its inhibitory effect on Aβ1-42 aggregation |
| 4 | Gold NP [211] | Real-time detection, dissolves aggregates and fibrils | 12 | Polyamidoamine (PAMAM) [212] | Contains sialic acid |
| 5 | Maghemite NP [213] | Rhodamine- and Congo-red-loaded NP: selective Aβ fibril labeling | 13 | G3 PAMAM dendrimer [214] | It improves its inhibitory action on Aβ1-28 aggregation at minimum doses |
| 6 | Conjugate polymer NP [215] | Direct interaction with Aβ: aggregation modulation | 14 | (NiPAM:BAM) NP [216] | Fibrillation of amyloid-β Retarded |
| 7 | Gold NP (13) [217] | NMDA functionalized: inhibits Aβ aggregation | 15 | Sulfonated, sulfated, and fluorinated PS NP [218] | Aβ oligomerization |
| 8 | NP liposome [219] | Planar curcumine: capture Aβ and reduces toxicity | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, N.A.; Alshamari, A.K.; Hassan, A.A.; Elharrif, M.G.; Alhajri, A.M.; Sattam, M.; Khattab, R.R. Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology. Molecules 2022, 27, 4839. https://doi.org/10.3390/molecules27154839
Hassan NA, Alshamari AK, Hassan AA, Elharrif MG, Alhajri AM, Sattam M, Khattab RR. Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology. Molecules. 2022; 27(15):4839. https://doi.org/10.3390/molecules27154839
Chicago/Turabian StyleHassan, Nasser A., Asma K. Alshamari, Allam A. Hassan, Mohamed G. Elharrif, Abdullah M. Alhajri, Mohammed Sattam, and Reham R. Khattab. 2022. "Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology" Molecules 27, no. 15: 4839. https://doi.org/10.3390/molecules27154839