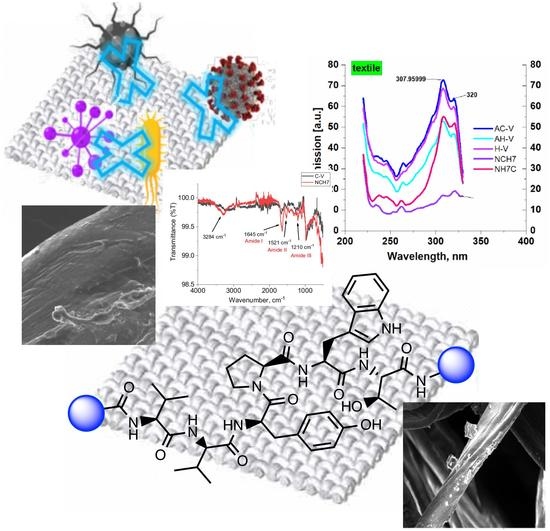
Study of Novel Peptides for Antimicrobial Protection in Solution and on Cotton Fabric
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Cotton Fabric Modification with Peptides
2.3. Physicochemical Characterizations
2.3.1. Spectral Characterizations
2.3.2. Electrochemical Characterizations
2.3.3. FTIR Analysis of Textile Materials
2.4. SEM Analysis of Textile Materials
2.5. Fastness Testing
2.6. Virological Activity
2.7. Antibacterial Activity
3. Materials and Methods
3.1. Synthesis of the Peptides (C-V, H-V, AC-V, AH-V, NH7C and NCH7)
3.2. Physicochemical Characterization
3.2.1. Spectral Measurements
Apparatus
Solutions for UV-Vis and Fluorescence Analysis
3.2.2. Electrochemical Measurements
3.3. Cotton Fabric Modification and Characterization
3.3.1. Modification of Cotton Fabric with Peptides
3.3.2. Fastness Testing
3.4. Virology
3.4.1. Cytotoxicity Assay
3.4.2. Antiviral Activity Assay
3.4.3. Virucidal Assay
3.5. Antibacterial Activity
3.5.1. Test Microorganisms
3.5.2. In Vitro Antimicrobial Assay
3.5.3. Antibacterial Activity of Cotton Fabrics Treated with the Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 2021, 12, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, A.; Abed, Y.; Boivin, G. August. Influenza drug resistance. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: Stuttgart, Germany, 2011; Volume 32, pp. 409–422. [Google Scholar]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of use of antiviral peptides against influenza virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef] [Green Version]
- Robinson, W.E., Jr.; McDougall, B.; Tran, D.; Selsted, M.E. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 1998, 63, 94–100. [Google Scholar] [CrossRef]
- Pazos, E.; Vazquez, O.; Mascarenas, J.L.; Vazquez, M.E. Peptide-based fluorescent biosensors. Chem. Soc. Rev. 2009, 38, 3348–3359. [Google Scholar] [CrossRef]
- Wang, L.; Xie, J.; Schultz, P.G. Expanding the genetic code. Annu. Rev. Biophys. Biomol. Struct. 2006, 35, 225–249. [Google Scholar] [CrossRef]
- Thurley, S.; Röglin, L.; Seitz, O. Hairpin peptide beacon: Dual-labeled PNA-peptide-hybrids for protein detection. J. Am. Chem. Soc. 2007, 129, 12693–12695. [Google Scholar] [CrossRef]
- Chersi, A.; Giommi, S.; Rosanò, L. Selective ‘in synthesis’ labeling of peptides with biotin and rhodamine. Biochim. Biophys. Acta. Gen. Subj. 2000, 1474, 196–200. [Google Scholar] [CrossRef]
- Orlandin, A.; Dolcet, P.; Biondi, B.; Hilma, G.; Coman, D.; Oancea, S.; Formaggio, F.; Peggion, C. Covalent Graft of Lipopeptides and Peptide Dendrimers to Cellulose Fibers. Coatings 2019, 9, 606. [Google Scholar] [CrossRef] [Green Version]
- Nyberg, F.; Sanderson, K.; Glämsta, E.L. The hemorphins: A new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers 1997, 43, 147–156. [Google Scholar] [CrossRef]
- Glämsta, E.L.; Marklund, A.; Hellman, U.; Wernstedt, C.; Terenius, L.; Nyberg, F. Isolation and characterization of a hemoglobinderived opioid peptide from the human pituitary gland. Regul. Pept. 1991, 34, 169–179. [Google Scholar] [CrossRef]
- Lammerich, H.P.; Busmann, A.; Kutzleb, C.; Wendland, M.; Seiler, P.; Berger, C.; Eickelmann, P.; Meyer, M.; Forssmann, W.G.; Maronde, E. Identification and functional characterization of hemorphins vv-h-7 and lvv-h-7 as low-affinity agonists for the orphan bombesin receptor subtype 3. Br. J. Pharmacol. 2003, 138, 1431–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkhudaryan, N. In vivo microdialysis is a tool to study the mechanism of interaction between lvv-hemorphin-7 and brain serotonergic system. Biotechnol. Health Yerevan 2005, 32–42. [Google Scholar]
- Mielczarek, P.; Hartman, K.; Drabik, A.; Hung, H.Y.; Huang, E.Y.K.; Gibula-Tarlowska, E.; Kotlinska, J.H.; Silberring, J. Hemorphins—From Discovery to Functions and Pharmacology. Molecules 2021, 26, 3879. [Google Scholar] [CrossRef]
- Blishchenko, E.Y.; Sazonova, O.V.; Kalinina, O.A.; Yatskin, O.N.; Philippova, M.M.; Surovoy, A.Y.; Karelin, A.A.; Ivanov, V.T. Family of hemorphins: Co-relations between amino acid sequences and effects in cell cultures. Peptides 2002, 23, 903–910. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Yoshikawa, M. Release of Hemorphin-5 from Human Hemoglobin by Pancreatic Elastase. Biosci. Biotechnol. Biochem. 2002, 66, 1130–1132. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Alzeyoudi, S.A.R.; Almutawa, S.A.; Alnajjar, A.N.; Vijayan, R. Molecular basis of the therapeutic properties of hemorphins. Pharmacol. Res. 2020, 158, 104855. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Georgieva, S.; Tchekalarova, J.; Vitkova, V.; Antonova, K.; Georgiev, A. Synthesis, characterization and anticonvulsant activity of new azobenzene-containing VV-hemorphin-5 bio photoswitch. Amino Acids 2019, 51, 549–563. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Rangelov, M.; Georgieva, S.; Todorova, N. Synthesis, characterization and anticonvulsant activity of new series of N-modified analogues of VV-hemorphin-5 with aminophosphonate moiety. Amino Acids 2019, 51, 1527–1545. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S. Potential anticonvulsant activity of novel VV-hemorphin-7 analogues containing unnatural amino acids: Synthesis and characterization. Amino Acids 2020, 52, 567–585. [Google Scholar] [CrossRef]
- Todorov, P.; Georgieva, S.; Peneva, P.; Tchekalarova, J. Spectral and electrochemical solvatochromic investigations of newly synthesized peptide-based chemosensor bearing azobenzene side chain bio photoswitch. Dyes Pigm. 2021, 191, 109348. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S.; Rangelov, M.; Todorova, N. Structure–activity relationship study on new Hemorphin-4 analogues containing steric restricted amino acids moiety for evaluation of their anticonvulsant activity. Amino Acids 2020, 52, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Peneva, P.; Pechlivanova, D.; Georgieva, S.; Dzhambazova, E. Synthesis, characterization and nociceptive screening of new VV-hemorphin-5 analogues. Bioorg. Med. Chem. Lett. 2018, 28, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Rangelov, M.; Peneva, P.; Todorova, N.; Tchekalarova, J. Anticonvulsant evaluation and docking analysis of VV-Hemorphin-5 analogues. Drug Dev. Res. 2019, 80, 425–437. [Google Scholar] [CrossRef]
- Ali, A.; Baby, B.; Soman, S.S.; Vijayan, R. Molecular insights into the interaction of hemorphin and its targets. Sci. Rep. 2020, 9, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Srivastava, N.; Garg, P.; Srivastava, P.; Seth, P.K. A molecular dynamics simulation study of the ACE2 receptor with screened natural inhibitors to identify novel drug candidate against COVID-19. PeerJ 2021, 9, e11171. [Google Scholar] [CrossRef] [PubMed]
- Mondal, I.H. (Ed.) Antimicrobial Textiles from Natural Resources; The Textile Institute Book Series; Woodhead Publishing: Cambridge, UK, 2021; pp. 45–85. [Google Scholar]
- Laird, K.; Riley, K. Antimicrobials Textiles; Sun, G., Ed.; Woodhead Publishing—Elsevier: Duxford, UK, 2016; pp. 249–262. [Google Scholar]
- Ballottin, D.; Fulaza, S.; Cabrini, F.; Tsukamoto, J.; Durán, N.; Alves, O.L.; Tasic, L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C 2017, 75, 582–589. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Grabchev, I. Synthesis of new modified with Rhodamine B peptides for antiviral protection of textile materials. Molecules 2021, 26, 6608. [Google Scholar] [CrossRef]
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The Lipophilic Bullet Hits the Targets: Medicinal Chemistry of Adamantane Derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [Green Version]
- Tencheva, A.; Liu, R.; Volkova, T.V. Synthetic analogues of memantine as neuroprotective and influenza viral inhibitors: In vitro and physicochemical studies. Amino Acids 2020, 52, 1559–1580. [Google Scholar] [CrossRef]
- Chowdhury, S.M.; Talukder, S.A.; Khan, A.M.; Afrin, N.; Ali, M.A.; Islam, R.; Parves, R.; Mamun, A.A.; Sufian, M.A.; Hossain, M.N.; et al. Antiviral Peptides as Promising Therapeutics against SARS-CoV-2. J. Phys. Chem. B 2020, 124, 9785–9792. [Google Scholar] [CrossRef]
- Opitakorn, A.; Rauytanapanit, M.; Waditee-Sirisattha, R.; Praneenararat, T. Non-leaching antibacterial cotton fabrics based on lipidated peptides. RSC Adv. 2017, 7, 34267–34275. [Google Scholar] [CrossRef] [Green Version]
- Mosbah, A.; Chouchane, H.; Abdelwahed, S.; Redissi, A.; Hamdi, M.; Kouidhi, S.; Neifar, M.; Masmoudi, A.S.; Cherif, A.; Mnif, W. Peptides Fixing Industrial Textile Dyes: A New Biochemical Method in Wastewater Treatment. Hindawi J. Chem. 2019, 2019, 5081807. [Google Scholar] [CrossRef] [Green Version]
- Shiryaev, V.A.; Klimochkin, Y.N. Heterocyclic inhibitors of viroporins in the design of antiviral compounds. Chem. Heterocycl. Compd. 2020, 56, 626–635. [Google Scholar] [CrossRef]
- Tai, K.P.; Le, V.V.; Selsted, M.E.; Ouellette, A.J. Hydrophobic determinants of α-defensin bactericidal activity. Infect. Immun. 2014, 82, 2195–2202. [Google Scholar] [CrossRef] [Green Version]
- Stevenazzi, A.; Marchini, M.; Sandrone, G.; Vergani, B.; Lattanzio, M. Amino acidic scaffolds bearing unnatural side chains: An old idea generates new and versatile tools for the life sciences. Bioorg. Med. Chem. Lett. 2014, 24, 5349–5356. [Google Scholar] [CrossRef] [Green Version]
- Adochitei, A.; Drochioiu, G. Rapid characterization of peptide secondary structure by ft-ir spectroscopy. Rev. Roum. Chim. 2011, 56, 783–791. [Google Scholar]
- Watkins, A.M.; Craven, T.W.; Renfrew, P.D.; Arora, P.S.; Bonneau, R. Rotamer Libraries for the High-Resolution Design of β-Amino Acid Foldamers. Structure 2017, 25, 1771–1780. [Google Scholar] [CrossRef] [Green Version]
- Fornander, L.H.; Feng, B.; Beke-Somfai, T.; Nordén, B. UV transition moments of tyrosine. J. Phys. Chem. B 2014, 118, 9247–9257. [Google Scholar] [CrossRef] [PubMed]
- Yashchuk, V.M.; Kudrya, V.Y.; Levchenko, S.M.; Tkachuk, Z.Y.; Hovorun, D.M.; Mel’nik, V.I.; Vorob’yov, V.P.; Klishevich, G.V. Optical response of the polynucleotides-proteins interaction. Mol. Cryst. Liq. Cryst. 2011, 535, 93–110. [Google Scholar] [CrossRef]
- Chen, R.F. Fluorescence Quantum Yields of Tryptophan and Tyrosine. Anal. Lett. 1967, 1, 35–42. [Google Scholar] [CrossRef]
- Alston, R.W.; Lasagna, M.; Grimsley, G.R.; Scholtz, J.M.; Reinhart, G.D.; Pace, C.N. Peptide Sequence and Conformation Strongly Influence Tryptophan Fluorescence. Biophys. J. 2008, 94, 2280–2287. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Protein Fluorescence. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 529–575. [Google Scholar] [CrossRef]
- Marsh, R.J.; Jones, R.A.L.; Sferrazza, M. Adsorption and displacement of a globular protein on hydrophilic and hydrophobic surfaces. Colloids Surf. B Biointerfaces 2002, 23, 31–42. [Google Scholar] [CrossRef]
- Haward, S.J.; Shewry, P.R.; Miles, M.J.; McMaster, T.J. Direct real-time imaging of protein adsorption onto hydrophilic and hydrophobic surfaces. Biopolymers 2010, 93, 74–84. [Google Scholar] [CrossRef]
- Wahlgren, M.; Arnebrant, T. Protein adsorption to solid surfaces. Trends Biotechnol. 1991, 9, 201–208. [Google Scholar] [CrossRef]
- Shen, L.; Huang, R.; Hu, N. Myoglobin in polyacrylamide hydrogel films: Direct electrochemistry and electrochemical catalysis. Talanta 2002, 56, 1131–1139. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Boron doped diamond and glassy carbon electrodes comparative study of the oxidation behaviour of cysteine and methionine. Bioelectrochemistry 2011, 81, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and Para-Substituted Phenols Electrochemical Oxidation Pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Pathways of Electrochemical Oxidation of Indolic Compounds. Electroanalysis 2011, 23, 1337–1344. [Google Scholar] [CrossRef]
- Chen, L.C.; Chang, C.C.; Chang, H.C. Electrochemical oxidation of histidine at an anodic oxidized boron-doped diamond electrode in neutral solution. Electrochim. Acta 2008, 53, 2883–2889. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brettl, A.M. Peptide methionine sulfoxide reductase A (MsrA): Direct electrochemical oxidation on carbon electrodes. Bioelectrochemistry 2013, 89, 11–18. [Google Scholar] [CrossRef]
- Gordon, S.; Hsieh, Y.L. Cotton: Science and Technology, 1st ed.; Woodhead Publishing Series in Textiles: Cambridge, UK, 2007; ISBN 978-1845690267/10-1845690265. [Google Scholar]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Sarin, V.K.; Kent, S.B.H.; Tam, J.P.; Merrifield, R.B. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal. Biochem. 1986, 117, 147–157. [Google Scholar] [CrossRef]
- Mohini, K.; Tejashree, L.; Vijay, N. Data set on analysis of dyeing property of natural dye from Thespesia populnea bark on different. Data Brief 2018, 16, 401–410. [Google Scholar] [CrossRef]















| Peptide Code | Peptide Structure | Molecular Formula | a [MH]+ Calculated | a [MH]+ Observed | b tR, min | c [α]54620 (°) |
|---|---|---|---|---|---|---|
| C-V |  | C47H66N10O12S | 994,4582 | 995,4631 | 18.75 | −54 |
| H-V |  | C50H68N12O12 | 1028,5080 | 1029,5148 | 16.85 | −40 |
| AC-V |  | C58H80N10O13S | 1156,5627 | 1157,5685 | 38.14 | −44 |
| AH-V |  | C61H82N12O13 | 1190,6124 | 1191,6147 | 29.26 | −44 |
| NH7C |  | C74H101N17O16S | 1515,7333 | 1516,7407 | 25.89 | −34 |
| NCH7 |  | C74H101N17O16S | 1515,7333 | 1516,7406 | 25.82 | −36 |
| Compounds | λabs [nm] | ε [L/(mol·cm)] | λem [nm] | Stokes Shift [cm−1] | Quantum Yield |
|---|---|---|---|---|---|
| AH-V | 295 | 6.62 × 105 | 356 | 5808 | 0.67 |
| C-V | 295 | 1.27 × 106 | 357 | 5887 | 0.24 |
| H-V | 295 | 1.30 × 106 | 356 | 5808 | 0.31 |
| AC-V | 295 | 6.12 × 105 | 354 | 5650 | 0.61 |
| NH7C | 295 | 7.49 × 105 | 355 | 5729 | 0.27 |
| NC7H | 295 | 1.44 × 105 | 355 | 5729 | 0.12 |
| Virus | Δlog 30 min | Δlog 60 min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-V | H-V | AC-V | AH-V | NH7C | NCH7 | C-V | H-V | AC-V | AH-V | NH7C | NCH7 | |
| HRSV-2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 1.1 | 1.6 | 1.4 | 1.3 | 1.0 | 0.9 |
| HAdV-5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Virus | Δlog 30 min | Δlog 60 min | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-V-Textile | H-V-Textile | AC-V-Textile | AH-V-Textile | NH7C-Textile | NCH7-Textile | C-V-Textile | H-V-Textile | AC-V-Textile | AH-V-Textile | NH7C-Textile | NCH7-Textile | |
| HRSV-2 | 0 | 0 | 0 | 0 | 0 | 0 | 0.9 | 1.0 | 1.0 | 0.9 | 0.8 | 0.6 |
| HAdV-5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Compound | Cytotoxicity |
|---|---|
| CC50 (µM/mL) in HEp-2 Cells | |
| C-V | 10.7 |
| H-V | 12.2 |
| AC-V | 110 |
| AH-V | 106 |
| NH7C | 170 |
| NCH7 | 139 |
| Ribavirin | 2103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorov, P.; Georgieva, S.; Staneva, D.; Peneva, P.; Grozdanov, P.; Nikolova, I.; Vasileva-Tonkova, E.; Grabchev, I. Study of Novel Peptides for Antimicrobial Protection in Solution and on Cotton Fabric. Molecules 2022, 27, 4770. https://doi.org/10.3390/molecules27154770
Todorov P, Georgieva S, Staneva D, Peneva P, Grozdanov P, Nikolova I, Vasileva-Tonkova E, Grabchev I. Study of Novel Peptides for Antimicrobial Protection in Solution and on Cotton Fabric. Molecules. 2022; 27(15):4770. https://doi.org/10.3390/molecules27154770
Chicago/Turabian StyleTodorov, Petar, Stela Georgieva, Desislava Staneva, Petia Peneva, Petar Grozdanov, Ivanka Nikolova, Evgenia Vasileva-Tonkova, and Ivo Grabchev. 2022. "Study of Novel Peptides for Antimicrobial Protection in Solution and on Cotton Fabric" Molecules 27, no. 15: 4770. https://doi.org/10.3390/molecules27154770