The Agronomic Traits, Alkaloids Analysis, FT-IR and 2DCOS-IR Spectroscopy Identification of the Low-Nicotine-Content Nontransgenic Tobacco Edited by CRISPR–Cas9
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Alkaloids Analysis
2.3. FT-IR and 2DCOS-IR Analysis
3. Results and Discussion
3.1. Effects of BBLs Knockout on Agronomic Traits
3.2. Effects of BBLs Knockout on Alkaloid Metabolism
3.3. FT-IR Analysis of Typical Tobacco Samples
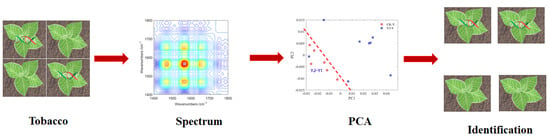
3.4. PCA Analysis of Roots and Leaves of Tobacco Samples
- (a)
- Let the optimal projection direction be w. Then, the principal component vector is obtained after projection:
- (b)
- The optimal projection direction should be such that the projection vector on it has the maximum differentiation, i.e., the optimization objective is to maximize the variance of t after projection; then, the problem is transformed to solve for the eigenvector w of the square matrix .
- (c)
- The projection X can be expressed in the form of a regression on the vector t:where p is the regression vector, E is the residual matrix, and t is the i-th principal component.
- (d)
- The residual matrix E is used as the new X. Repeat the process of (a)~(c) until the first R principal components are all determined.
- (e)
- After the first R principal components are selected, all vectors t form the principal component matrix T to complete the data dimensionality reduction.
3.5. 2DCOS-IR Spectroscopy and PCA Classification of Tobacco Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guo, Y.; Hiatt, E.; Bonnet, C.; Kudithipudi, C.; Lewis, R.S.; Shi, H.; Patra, B.; Zhao, X.; de Borne, F.D.; Gilles, T.; et al. Molecular regulation and genetic manipulation of alkaloid accumulation in tobacco plants. Stud. Bioact. Nat. Prod. 2021, 70, 119–149. [Google Scholar] [CrossRef]
- Kajikawa, M.; Sierro, N.; Kawaguchi, H.; Bakaher, N.; Ivanov, N.V.; Hashimoto, T.; Shoji, T. Genomic Insights into the Evolution of the Nicotine Biosynthesis Pathway in Tobacco. Plant Physiol. 2017, 174, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites—Pathways, transcription factors and applied aspects—A brief review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Hirai, N.; Hashimoto, T. A PIP-family protein is required for biosynthesis of tobacco alkaloids. Plant Mol. Biol. 2009, 69, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Wang, W.; Niu, H.; Ding, Y.; Zhang, D.; Zhang, J.; Liu, G.; Wang, S.; Zhang, H. Jasmonate-Sensitivity-Assisted Screening and Characterization of Nicotine Synthetic Mutants from Activation-Tagged Population of Tobacco (Nicotiana tabacum L.). Front. Plant Sci. 2017, 8, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Lewis, R.S.; Shi, J.; Song, Z.; Gao, Y.; Li, W.; Chen, H.; Qu, R. Genetic Factors for Enhancement of Nicotine Levels in Cultivated Tobacco. Sci. Rep. 2015, 5, 17360. [Google Scholar] [CrossRef] [PubMed]
- Schachtsiek, J.; Stehle, F. Nicotine-free, nontransgenic tobacco (Nicotiana tabacum L.) edited by CRISPR-Cas9. Plant Biotechnol. J. 2019, 17, 2228–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowski, L.T. Cigarette prohibition and the need for more prior testing of the WHO TobReg’s global nicotine-reduction strategy. Tob. Control 2017, 26, e31–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.S.; Lopez, H.O.; Bowen, S.W.; Andres, K.R.; Steede, W.T.; Dewey, R.E. Transgenic and mutation-based suppression of a berberine bridge enzyme-like (BBL) gene family reduces alkaloid content in field-grown tobacco. PLoS ONE 2015, 10, e0117273. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.; Yuliana, N.D.; Simanjuntak, P.; Rafi, M.; Abdillah, S. FTIR and HPLC-Based Metabolomics of Yacon Leaves Extracts (Smallanthus sonchifolius [Poepp & Endl.] H. Robinson) from Two Locations in Indonesia. Indones J. Chem. 2020, 20, 567–578. [Google Scholar] [CrossRef]
- Favaro, L.; Corte, L.; Roscini, L.; Cagnin, L.; Tiecco, M.; Colabella, C.; Berti, A.; Basaglia, M.; Cardinali, G.; Casella, S. A novel FTIR-based approach to evaluate the interactions between lignocellulosic inhibitory compounds and their effect on yeast metabolism. RSC Adv. 2016, 6, 47981–47989. [Google Scholar] [CrossRef]
- Yunusa, A.K.; Rashid, Z.M.; Mat, N.; Abu Bakar, C.A.; Ali, A.M. Chemicals and Bioactivity Discrimination of Syconia of Seven Varieties of Ficus deltoidea Jack via ATR-IR Spectroscopic-Based Metabolomics. Pharmacogn. J. 2018, 10, s147–s151. [Google Scholar] [CrossRef] [Green Version]
- Noda, I. Two-dimensional infrared (2D IR) spectroscopy: Theory and applications. Appl. Spectrosc. 1990, 44, 550–561. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons, Ltd.: Tokyo, Japan, 2005; pp. 10–45. [Google Scholar]
- Ma, F.; Huang, A.-m. Rapid identification and quantification three chicken-wing woods of Millettia leucantha, Millettia laurentii and Cassia siamea by FT-IR and 2DCOS-IR. J. Mol. Struct. 2018, 1166, 164–168. [Google Scholar] [CrossRef]
- Popescu, C.M.; Jones, D.; Kržišnik, D.; Humar, M. Determination of the effectiveness of a combined thermal/chemical wood modification by the use of FT–IR spectroscopy and chemometric methods. J. Mol. Struct. 2020, 1200, 127133. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J.; Zhou, Q.; Lu, G.; Chan, K. Application of mid-infrared spectroscopy in the quality control of traditional Chinese medicines. Planta Med. 2010, 76, 1987–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.-Q.; Zhou, Q.; Qin, Z. Atlas of Two-Dimensional Correlation Infrared Spectroscopy for Traditional Chinese Medicine Identification; Chemical Industry Press: Beijing, China, 2003; pp. 9–35. [Google Scholar]
- Sun, S.Q.; Zhou, Q.; Chen, J.-B. Infrared Spectroscopy for Complex Mixtures: Applications in Food and Traditional Chinese Medicine; Chemical Industry Press: Beijing, China, 2011; pp. 1–20. [Google Scholar]
- YC/T 559-2018; Characteristic Components of Tobacco-Determination of Alkaloids-Gas Chromatography-Mass Spectrometry Method and Gas Chromatography-Tandem Mass Spectrometry Method. China National Tobacco Corporation: Beijing, China, 2018.
- YC/T 142-2010; Investigation and Measurement Methods of Agronomic Characters of Tobacco. China National Tobacco Corporation: Beijing, China, 2010.
- Jiang, Z.; Yi, J.; Li, J.; He, T.; Hu, C. Promoting Effect of Sodium Chloride on the Solubilization and Depolymerization of Cellulose from Raw Biomass Materials in Water. ChemSusChem 2015, 8, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Largo-Gosens, A.; Hernández-Altamirano, M.; García-Calvo, L.; Alonso-Simón, A.; Álvarez, J.; Acebes, J.L. Fourier transform mid infrared spectroscopy applications for monitoring the structural plasticity of plant cell walls. Front. Plant Sci. 2014, 5, 303. [Google Scholar] [CrossRef] [Green Version]
- Popescu, M.-C.; Froidevaux, J.; Navi, P.; Popescu, C.-M. Structural modifications of Tilia cordata wood during heat treatment investigated by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2013, 1033, 176–186. [Google Scholar] [CrossRef]
- Goodfellow, I.; Bengio, Y.; Courville, A. Deep Learning; MIT Press: Boston, MA, USA, 2016; pp. 12–37. [Google Scholar]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Analysis Part | Code Name | Sample Numbers | |
|---|---|---|---|
| Hongda wild-type tobacco (CK) | Root | CK-G | 8 |
| Leaf | CK-Y | 6 | |
| Gene knockout mutants (YJ) | Root | YJ-G | 6 |
| Leaf | YJ-Y | 8 |
| ID | PH (cm) | GS (cm) | LTL (cm) | WTL (cm) | NL | |
|---|---|---|---|---|---|---|
| Max | 130.0 | 14.0 | 68.0 | 25.0 | 15.0 | |
| YJ | Ave | 126.8 | 13.3 | 61.4 | 24.0 | 14.8 |
| Min | 110.0 | 12.8 | 56.0 | 19.0 | 14.0 | |
| Max | 134.0 | 13.8 | 67.0 | 28.0 | 16.0 | |
| CK | Ave | 130.8 | 13.4 | 65.8 | 25.0 | 15.2 |
| Min | 128.0 | 11.8 | 62.0 | 24.0 | 15.0 | |
| Peak Position/cm−1 | Functional Group | Main Attribution | |
|---|---|---|---|
| Root | Leaf | ||
| 3389 | 3374 | sν (O−H, N−H) | OH, NH |
| 2928 | 2928 | νas (CH2) | CH2 |
| 1628 | 1621 | ν (C=O−O), ν (Ar) | calcium oxalate, carboxyl |
| - | 1416 | δ (CH2), δ (C−O−H) | lignin, cellulose |
| 1375 | - | νas (C−N−C), δ (C−O−H) | lignin, cellulose |
| 1317 | 1317 | ν (C−O−H) | calcium oxalate |
| 1153 | 1153 | ν (C−C,C−O), δ (C−O−H) | cellulose |
| 1077 | 1079 | ν (C−C,C−O), δ (C−O−H) | lignin |
| 1031 | 1039 | ν (C−C,C−O), δ (C−O−H) | cellulose |
| 850 | ν (C−C,C−O), δ (C−O−H) | cellulose | |
| 781 | 781 | ν (C−C) | calcium oxalate |
| 518 | - | C−O−H | calcium oxalate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhou, Q.; Zhang, D.; Yang, G.; Zhang, C.; Wu, Y.; Xu, Y.; Chen, J.; Kong, W.; Kong, G.; et al. The Agronomic Traits, Alkaloids Analysis, FT-IR and 2DCOS-IR Spectroscopy Identification of the Low-Nicotine-Content Nontransgenic Tobacco Edited by CRISPR–Cas9. Molecules 2022, 27, 3817. https://doi.org/10.3390/molecules27123817
Zhang J, Zhou Q, Zhang D, Yang G, Zhang C, Wu Y, Xu Y, Chen J, Kong W, Kong G, et al. The Agronomic Traits, Alkaloids Analysis, FT-IR and 2DCOS-IR Spectroscopy Identification of the Low-Nicotine-Content Nontransgenic Tobacco Edited by CRISPR–Cas9. Molecules. 2022; 27(12):3817. https://doi.org/10.3390/molecules27123817
Chicago/Turabian StyleZhang, Jianduo, Qun Zhou, Dongheyu Zhang, Guangyu Yang, Chengming Zhang, Yuping Wu, Yong Xu, Jianhua Chen, Weisong Kong, Guanghui Kong, and et al. 2022. "The Agronomic Traits, Alkaloids Analysis, FT-IR and 2DCOS-IR Spectroscopy Identification of the Low-Nicotine-Content Nontransgenic Tobacco Edited by CRISPR–Cas9" Molecules 27, no. 12: 3817. https://doi.org/10.3390/molecules27123817