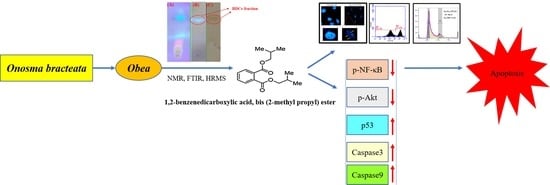
Targeting Akt/NF-κB/p53 Pathway and Apoptosis Inducing Potential of 1,2-Benzenedicarboxylic Acid, Bis (2-Methyl Propyl) Ester Isolated from Onosma bracteata Wall. against Human Osteosarcoma (MG-63) Cells
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity
2.1.1. Superoxide Anion Radical Scavenging Assay
2.1.2. Lipid Peroxidation Assay
2.2. Identification and Isolation of Bioactive Compound
2.2.1. Isolation of Pure Compound from Silica Gel Chromatography
2.2.2. Characterization and Structure Elucidation of BDCe Rraction
2.3. Antiproliferative Activity BDCe Fraction
2.4. Morphological Change Visualized in MG-63 Cell Line
2.4.1. Scanning Electron Microscopy (SEM) Studies Confirm Apoptotic Cell Death
2.4.2. Dual AO/EtBr Staining
2.4.3. BDCe Fraction Alter Mitochondria Membrane Potential (ΔΨm)
2.4.4. Hoechst Staining
2.4.5. In Vitro Cell Scratch Migration Assay
2.5. Flow Cytometric Analysis
2.5.1. ROS Analysis
2.5.2. Measurement of MMP (ΔΨm) Analysis
2.5.3. Cell Cycle Distribution
2.6. Western Blotting
2.7. RT-qPCR Analysis
2.8. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Plant Procurement, Identification, and Authentication of Plant Material
4.3. Extraction and Fractionation
4.4. Antioxidant Activity
4.4.1. Superoxide Anion Radical Scavenging Assay
4.4.2. Lipid Peroxidation Assay
4.5. Column Chromatography of the Obea Fraction
4.5.1. Isolation of BDCe Fraction
4.5.2. Structure Elucidation and Characterization of the BDCe Fraction
4.6. Cell Culture
4.7. MTT Assay
4.8. Assessment of Cells’ Microscopic Studies
4.8.1. Morphological Changes of MG-63 Cells under Phase-Contrast Microscope
4.8.2. Scanning Electron Microscopy (SEM)
4.8.3. Fluorescence Microscopy
Dual Acridine Orange (AO) and Ethidium Bromide (EtBr) (AO/EtBr) Staining
Determination of Mitochondrial Membrane Potential (MMP)
Hoechst Staining
4.9. Cell Migration Assay
4.10. Flow Cytometric Studies
4.10.1. ROS Generation Analysis
4.10.2. Mitochondria Membrane Potential (MMP) Analysis
4.10.3. Cell-Cycle Phase Distribution Analysis
4.11. Western Blotting
4.12. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.13. Molecular Docking Studies
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The Osteosarcoma Microenvironment: A Complex but Targetable Ecosystem. Cells 2020, 9, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A.A. Osteosarcoma: A comprehensive review. SICOT-J. 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, J.; Zeng, J.; Song, C.; Yu, H.; Shi, Q.; Mai, W.; Qu, G. A Retrospective Clinicopathological Study of Osteosarcoma Patients with Metachronous Metastatic Relapse. J. Cancer 2019, 10, 2982–2990. [Google Scholar] [CrossRef] [Green Version]
- Siamof, C.M.; Goel, S.; Cai, W. Moving Beyond the Pillars of Cancer Treatment: Perspectives From Nanotechnology. Front. Chem. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Bielack, S.; Carrle, D.; Casali, P.G.; ESMO Guidelines Working Group. Osteosarcoma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann. Oncol. 2009, 20, iv137–iv139. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Loeb, K.R.; Loeb, L.A. Significance of multiple mutations in cancer. Carcinogenesis 2000, 21, 379–385. [Google Scholar] [CrossRef]
- Li, B.; Dou, S.-X.; Yuan, J.-W.; Liu, Y.-R.; Li, W.; Ye, F.; Wang, P.-Y.; Li, H. Intracellular transport is accelerated in early apoptotic cells. Proc. Natl. Acad. Sci. USA 2018, 115, 12118–12123. [Google Scholar] [CrossRef] [Green Version]
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-κB Pathway and Cancer Stem Cells. Cells 2016, 5, 16. [Google Scholar] [CrossRef]
- Bassères, D.S.; Baldwin, A.S. Nuclear factor-κB and inhibitor of κB kinase pathways in oncogenic initiation and progression. Oncogene 2006, 25, 6817–6830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.G.; Park, J.; Park, J.Y.; Park, C.H.; Lee, K.-H.; Cho, J.W.; Hwang, J.-I.; Seong, J.Y. Anti-Cancer Activity of a Novel Small Molecule Compound That Simultaneously Activates p53 and Inhibits NF-κB Signaling. PLoS ONE 2012, 7, e44259. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, A.; Shebaby, W.; Nehmeh, B.; Faour, W.; Bassil, B.; El Hakim, J.; Iskandar, R.; Dib-Jalbout, N.; Mroueh, M.; Daher, C.; et al. In Vitro and In Vivo Evaluation of the Anticancer and Anti-inflammatory Activities of 2-Himachelen-7-ol isolated from Cedrus Libani. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.-R.; Zhou, W.; Zhang, H.-M.; Guo, Q.-S.; Yang, W.; Li, B.-J.; Sun, Z.-H.; Gao, S.-H.; Cui, R.-J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef] [Green Version]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M. Essential oil composition of Anchusa italica from Iran. Chem. Nat. Compd. 2013, 49, 369–370. [Google Scholar] [CrossRef]
- Ved, D.K.; Sureshchandra, S.T.; Barve, V.; Srinivas, V.; Sangeetha, S.; Ravikumar, K.; Kartikeyan, R.; Kulkarni, V.; Kumar, A.S.; Venugopal, S.N.; et al. Bengaluru: FRLHT’s ENVIS Centre on Medicinal Plants. 2016, pp. 1475–1484. Available online: https://envis.frlht.org/frlhtenvis.nic.in (accessed on 13 December 2020).
- Zeb, M.A.; Sajid, M.; Rahman, T.U.; Khattak, K.F.; Halim, M. Phytochemical Screening and Antibacterial Activity of Opuntia dillenii and Onosma bracteatum. J. Microbiol. Exp. 2015, 3, 216–219. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kishore, K.; Onosma, L. A review of phytochemistry and ethnopharmacology. Pharmacogn. Rev. 2013, 7, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Albaqami, J.; Myles, L.E.; Tiriveedhi, V. The Effect of Onosma bracteatum in cancer cells. MOJ Bioequiv. Availab. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Farooq, U.; Pan, Y.; Disasa, D.; Qi, J. Novel Anti-Aging Benzoquinone Derivatives from Onosma bracteatum Wall. Molecules 2019, 24, 1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Kaur, S.; Pandit, K.; Kaur, V.; Thakur, S.; Kaur, S. Onosma bracteata Wall. induces G0/G1 arrest and apoptosis in MG-63 human osteosarcoma cells via ROS generation and AKT/GSK3β/cyclin E pathway. Environ. Sci. Pollut. Res. 2021, 28, 14983–15004. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y.-C. Sulfonated graphene as highly efficient and reusable acid carbocatalyst for the synthesis of ester plasticizers. RSC Adv. 2014, 4, 57297–57307. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Withania somnifera as a Potential Anxiolytic and Anti-inflammatory Candidate Against Systemic Lipopolysaccharide-Induced Neuroinflammation. NeuroMolecular Med. 2018, 20, 343–362. [Google Scholar] [CrossRef]
- Kumar, M.; Kaur, P.; Kumar, S.; Kaur, S. Antiproliferative and Apoptosis Inducing Effects of Non-Polar Fractions from Lawsonia inermis L. in Cervical (HeLa) Cancer Cells. Physiol. Mol. Biol. Plants 2015, 21, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.; Dai, C.; Cao, P.; Sun, D.; Ouyang, R.; Miao, Y. The role of reactive oxygen species in tumor treatment. RSC Adv. 2020, 10, 7740–7750. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [Green Version]
- Gali-Muhtasib, H.; Hmadi, R.A.; Kareh, M.; Tohme, R.; Darwiche, N. Cell death mechanisms of plant-derived anticancer drugs: Beyond apoptosis. Apoptosis 2015, 20, 1531–1562. [Google Scholar] [CrossRef]
- Shi, L.; Qin, H.; Jin, X.; Yang, X.; Lu, X.; Wang, H.; Wang, R.; Yu, D.; Feng, B. The natural phenolic peperobtusin A induces apoptosis of lymphoma U937 cells via the Caspase dependent and p38 MAPK signaling pathways. Biomed. Pharmacother. 2018, 102, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllősi, J.; Mocanu, M.-M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatiwora, E.; Adsula, V.B.; Kulkarni, M.; Deshpande, N.R.; Kashalkar, R.V. Isolation and characterization of substituted dibutyl phthalate from Ipomoea carnea stem. Der. Pharma. Chemica. 2013, 5, 5–10. [Google Scholar]
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef]
- Smaoui, S.; Mathieu, F.; Elleuch, L.; Coppel, Y.; Merlina, G.; Karray-Rebai, I.; Mellouli, L. Taxonomy, purification and chemical characterization of four bioactive compounds from new Streptomyces sp. TN256 strain. World J. Microbiol. Biotechnol. 2011, 28, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Anju, K.M.; Archana, M.M.; Mohandas, C.; Nambisan, B.A.L.A. An antimicrobial phthalate derivative from Bacillus cereus, the symbiotic bacterium associated with a novel entomopathogenic nematode, Rhabditis (Oscheius) sp. Int. J. Pharm. Pharm. Sci. 2015, 7, 238–242. [Google Scholar]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, C.; Yu, Y.; Liu, H.; Shen, Y. Distribution characteristics and health risk assessment of PAEs in urban soils of Changji City, Xinjiang, China. China J. Nat. 2020, 22, 67–74. [Google Scholar]
- Roy, R.N. Bioactive natural derivatives of phthalate ester. Crit. Rev. Biotechnol. 2020, 40, 913–929. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gao, W.; Wang, H. Study on chemical composition, anti-inflammatory and anti-microbial activities of extracts from Chinese pear fruit (Pyrus bretschneideri Rehd.). Food Chem. Toxicol. 2012, 50, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wu, Y.; Ning, D.; Wei, Y. Chemical constituents from the root of Croton lachynocarpus. Guangxi Zhiwu/Guihaia. 2014, 34, 148–150. [Google Scholar]
- Dong, S.J.; Bi, X.D.; Wang, N.; Song, L.; Dai, W.; Zhang, S.L. Algicidal activities of Cladophora fracta on red tide-forming microalgae Heterosigma akashiwo and Gymnodinium breve. Allelopath. J. 2016, 37, 231–240. [Google Scholar]
- Perveen, I.; Raza, M.; Iqbal, T.; Naz, I.; Sehar, S.; Ahmed, S. Isolation of anticancer and antimicrobial metabolites from Epicoccum nigrum; endophyte of Ferula sumbul. Microb. Pathog. 2017, 110, 214–224. [Google Scholar] [CrossRef]
- Mašković, P.Z.; Diamanto, L.D.; Vujic, J.M.; Cvetanović, A.D.; Radojković, M.M.; Gadžurić, S.B.; Zengin, G. Onosma aucheriana: A source of biologically active molecules for novel food ingredients and pharmaceuticals. J. Funct. Foods 2015, 19, 479–486. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Kosem, N.; Kaslungka, S.; Luanratana, O.; Pongpan, N.; Neungton, N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. J. Ethnopharmacol. 2003, 90, 161–166. [Google Scholar] [CrossRef]
- Li, S.-W.; Hu, K.-Z.; Chen, S.-C.; Liu, S.-L.; Wang, Y.-H. High expression of long non-coding RNA LOC730101 correlates with distant metastasis and exhibits a poor prognosis in patients with osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4115–4120. [Google Scholar]
- Wang, H.; Liu, Z.; Li, X.; Zhao, R.; Pu, Y.; Wu, H.; Guan, W. Shikonin causes apoptosis by disrupting intracellular calcium homeostasis and mitochondrial function in human hepatoma cells. Exp. Ther. Med. 2017, 15, 1484–1492. [Google Scholar] [CrossRef] [Green Version]
- Plava, J.; Cihova, M.; Burikova, M.; Matuskova, M.; Kucerova, L.; Miklikova, S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol. Cancer 2019, 18, 1–10. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma Cells. Cell. Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundaković, T.; Stanojković, T.; Juranić, Z.; Kovačević, N. Cytotoxicityin vitro of naphthazarin derivatives fromOnosma arenaria. Phytotherapy Res. 2006, 20, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Ukwubile, C.; Ikpefan, E.; Malgwi, T.; Bababe, A.; Odugu, J.; Angyu, A.; Otalu, O.; Bingari, M.; Nettey, H. Cytotoxic effects of new bioactive compounds isolated from a Nigerian anticancer plant Melastomastrum capitatum Fern. leaf extract. Sci. Afr. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Klotz, L.-O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 7, 2346. [Google Scholar] [CrossRef] [Green Version]
- Dilshara, M.G.; Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Kang, C.-H.; Jeong, J.-W.; Choi, Y.H.; Kim, G.-Y. β-Hydroxyisovalerylshikonin promotes reactive oxygen species production in HCT116 colon cancer cells, leading to caspase-mediated apoptosis. Rev. Bras. Farm. 2018, 28, 344–351. [Google Scholar] [CrossRef]
- Chan, S.-F.; Chen, Y.-Y.; Lin, J.-J.; Liao, C.-L.; Ko, Y.-C.; Tang, N.-Y.; Kuo, C.-L.; Liu, K.-C.; Chung, J.-G. Triptolide induced cell death through apoptosis and autophagy in murine leukemia WEHI-3 cellsin vitroand promoting immune responses in WEHI-3 generated leukemia mice in vivo. Environ. Toxicol. 2016, 32, 550–568. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, V.; Pandit, K.; Tuli, H.S.; Sak, K.; Jain, S.K.; Kaur, S. Antioxidant Phytoconstituents From Onosma bracteata Wall. (Boraginaceae) Ameliorate the CCl4 Induced Hepatic Damage: In Vivo Study in Male Wistar Rats. Front. Pharmacol. 2020, 11, 1–22. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, A.; Thakur, S.; Kumar, K.; Sharma, R.; Sharma, A.; Singh, P.; Sharma, U.; Kumar, S.; Landi, M.; et al. Antioxidant, Antiproliferative and Apoptosis-Inducing Efficacy of Fractions from Cassia fistula L. Leaves. Antioxidants 2020, 9, 173. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Sun, Y.; Zheng, C.; Hou, T.; Kang, M.; Yang, X. p53 mediated apoptosis in osteosarcoma MG-63 cells by inhibition of FANCD2 gene expression. Int. J. Clin. Exp. Med. 2015, 8, 11101–11108. [Google Scholar] [PubMed]
- Amin, A.R.; Karpowicz, P.A.; Carey, T.; Arbiser, J.; Nahta, R.; Chen, Z.G.; Dong, J.-T.; Kucuk, O.; Khan, G.N.; Huang, G.; et al. Evasion of anti-growth signaling: A key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. Semin. Cancer Biol. 2015, 35, S55–S77. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Di Agostino, S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J. Exp. Clin. Cancer Res. 2018, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amalarasi, L.E.; Jothi, G.J. Phytochemical profiling and antioxidant activity of the extracts of Pachygone ovata (Poir.) Miers ex Hook.f. & Thomson. J. Pharmacogn. Phytochem. 2019, 8, 204–209. [Google Scholar] [CrossRef]
- Qiao, Q.; Jiang, Y.; Li, G. Inhibition of the PI3K/AKT-NF-κB Pathway With Curcumin Enhanced Radiation-Induced Apoptosis in Human Burkitt’s Lymphoma. J. Pharmacol. Sci. 2013, 121, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.; Shang, X.; Ni, Z.; Shi, G. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis. Exp. Ther. Med. 2016, 12, 2735–2740. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yang, L.; Lu, Y.; Chen, Y.; Liu, T.; Peng, Y.; Zhou, Y.; Cao, Y.; Bi, Z.; Liu, T.; et al. Osthole Induces Cell Cycle Arrest and Inhibits Migration and Invasion via PTEN/Akt Pathways in Osteosarcoma. Cell. Physiol. Biochem. 2016, 38, 2173–2182. [Google Scholar] [CrossRef]
- Yan, M.; Xu, Q.; Zhang, P.; Zhou, X.-J.; Zhang, Z.-Y.; Chen, W.-T. Correlation of NF-κB signal pathway with tumor metastasis of human head and neck squamous cell carcinoma. BMC Cancer 2010, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Noori, A.-R.; Tashakor, A.; Nikkhah, M.; Eriksson, L.A.; Hosseinkhani, S.; Fearnhead, H.O. Loss of WD2 subdomain of Apaf-1 forms an apoptosome structure which blocks activation of caspase-3 and caspase. Biochimie 2020, 180, 23–29. [Google Scholar] [CrossRef]
- Jannus, F.; Medina-O’Donnell, M.; Rivas, F.; Díaz-Ruiz, L.; Rufino-Palomares, E.E.; Lupiáñez, J.A.; Parra, A.; Reyes-Zurita, F.J. A Diamine-PEGylated Oleanolic Acid Derivative Induced Efficient Apoptosis through a Death Receptor and Mitochondrial Apoptotic Pathway in HepG2 Human Hepatoma Cells. Biomolecules 2020, 10, 1375. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Wang, C.-J.; Chang, Y.-C.; Hung, T.-W.; Lai, C.-J.; Kuo, C.-W.; Huang, H.-P. Mulberry fruits extracts induce apoptosis and autophagy of liver cancer cell and prevent hepatocarcinogenesis in vivo. J. Food Drug Anal. 2019, 28, 84–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement. Altern. Med. 2015, 15, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ren, W.; Hu, W.-X. Apoptosis of HL-60 cells induced by extracts from Narcissus tazetta var. chinensis. Cancer Lett. 2006, 242, 133–140. [Google Scholar] [CrossRef]
- Rello-Varona, S.; Stockert, J.C.; Moreno, V.; Pacheco, M.; Juarranz, A.; Villanueva, A. Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments. Apoptosis 2005, 10, 201–208. [Google Scholar] [CrossRef]
- Banda, N.K.; Satterfield, W.C.; Dunlap, A.; Steimer, K.S.; Kurrle, R.; Finkel, T.H. Lack of gp120-induced anergy and apoptosis in chimpanzees is correlated with resistance to AIDS. Apoptosis 1996, 1, 49–62. [Google Scholar] [CrossRef]
- Devari, S.; Jaglan, S.; Kumar, M.; Deshidi, R.; Guru, S.; Bhushan, S.; Kushwaha, M.; Gupta, A.; Gandhi, S.G.; Sharma, J.P.; et al. Capsaicin production by Alternaria alternata, an endophytic fungus from Capsicum annum; LC–ESI–MS/MS analysis. Phytochemistry 2014, 98, 183–189. [Google Scholar] [CrossRef]
- Woo, M.; Hakem, R.; Soengas, M.; Duncan, G.S.; Shahinian, A.; Kägi, D.; Hakem, A.; McCurrach, M.; Khoo, W.; Kaufman, S.A.; et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998, 12, 806–819. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Kataria, H.; Kaul, S.C.; Ishii, T.; Kaur, G.; Wadhwa, R. Effect of the alcoholic extract of Ashwagandha leaves and its components on proliferation, migration, and differentiation of glioblastoma cells: Combinational approach for enhanced differentiation. Cancer Sci. 2009, 100, 1740–1747. [Google Scholar] [CrossRef]
- Deeb, D.; Gao, X.; Jiang, H.; Janic, B.; Arbab, A.S.; Rojanasakul, Y.; Dulchavsky, S.A.; Gautam, S.C. Oleanane triterpenoid CDDO-Me inhibits growth and induces apoptosis in prostate cancer cells through a ROS-dependent mechanism. Biochem. Pharmacol. 2010, 79, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajaniradje, S.; Mohankumar, K.; Pamidimukkala, R.; Subramanian, S.; Rajagopalan, R. Antiproliferative and Apoptotic Effects ofSesbania grandifloraLeaves in Human Cancer Cells. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Piovesan, D.; Minervini, G.; Tosatto, S.C. The RING 2.0 web server for high quality residue interaction networks. Nucleic Acids Res. 2016, 44, W367–W374. [Google Scholar] [CrossRef] [PubMed]



















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Kaur, S.; Dhiman, S.; Singh, P.P.; Bhatia, G.; Thakur, S.; Tuli, H.S.; Sharma, U.; Kumar, S.; Almutary, A.G.; et al. Targeting Akt/NF-κB/p53 Pathway and Apoptosis Inducing Potential of 1,2-Benzenedicarboxylic Acid, Bis (2-Methyl Propyl) Ester Isolated from Onosma bracteata Wall. against Human Osteosarcoma (MG-63) Cells. Molecules 2022, 27, 3478. https://doi.org/10.3390/molecules27113478
Kumar A, Kaur S, Dhiman S, Singh PP, Bhatia G, Thakur S, Tuli HS, Sharma U, Kumar S, Almutary AG, et al. Targeting Akt/NF-κB/p53 Pathway and Apoptosis Inducing Potential of 1,2-Benzenedicarboxylic Acid, Bis (2-Methyl Propyl) Ester Isolated from Onosma bracteata Wall. against Human Osteosarcoma (MG-63) Cells. Molecules. 2022; 27(11):3478. https://doi.org/10.3390/molecules27113478
Chicago/Turabian StyleKumar, Ajay, Sandeep Kaur, Sukhvinder Dhiman, Prithvi Pal Singh, Gaurav Bhatia, Sharad Thakur, Hardeep Singh Tuli, Upendra Sharma, Subodh Kumar, Abdulmajeed G. Almutary, and et al. 2022. "Targeting Akt/NF-κB/p53 Pathway and Apoptosis Inducing Potential of 1,2-Benzenedicarboxylic Acid, Bis (2-Methyl Propyl) Ester Isolated from Onosma bracteata Wall. against Human Osteosarcoma (MG-63) Cells" Molecules 27, no. 11: 3478. https://doi.org/10.3390/molecules27113478