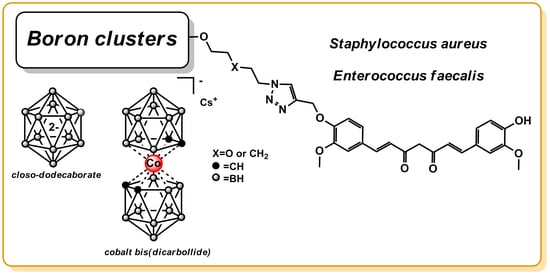
Synthesis and Antibacterial Activity Studies of the Conjugates of Curcumin with closo-Dodecaborate and Cobalt Bis(Dicarbollide) Boron Clusters †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Conjugates of Curcumin with closo-Dodecaborate and Cobalt Bis(Bicarbollide) Boron Clusters
2.2. Antibacterial Activity Studies
3. Materials and Methods
3.1. General Methods
General Procedure for the Synthesis of the Conjugates of Cobalt Bis(Dicarbollide) with Curcumin 3 and 4
3.2. Synthesis of (8-[(H(CH2[COCH=CH(OCH3)C6H3O]2))-CH2-C-CH-N3((CH2)2O)2]-3,3′-Co(1,2-C2B9H10)(1′,2′-C2B9H11))Cs 4
3.3. Synthesis of (8-[(H(CH2[COCH=CH(OCH3)C6H3O]2))-CH2-C-CH-N3(CH2)5O]-3,3′-Co(1,2-C2B9H10)(1′,2′-C2B9H11))Cs 5
General Procedure for the Synthesis of the Conjugates of closo-Dodecaborate with Curcumin 9–11
3.4. Synthesis of [(H(CH2[COCH=CH(OCH3)C6H3O]2))-CH2-C-CH-N3((CH2)2O)2]-(B12H11)]Cs2 9
3.5. Synthesis of [(H(CH2[COCH=CH(OCH3)C6H3O]2))-CH2-C-CH-N3(CH2)5O]-(B12H11)]Cs2 10
3.6. Synthesis of [(H(CH2[COCH=CH(OCH3)C6H3O]2))-CH2-C-CH-N3(CH2)4O]-(B12H11)]Cs2 11
3.7. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sivaev, I.B. Functional group directed B-H activation of polyhedral boron hydrides by transition metal complexes (review). Russ. J. Inorg. Chem. 2021, 66, 1289–1342. [Google Scholar] [CrossRef]
- King, R.B. Three-dimensional aromaticity in polyhedral boranes and related molecules. Chem. Rev. 2001, 101, 1119–1152. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; King, R.B. Spherical aromaticity: Recent work on fullerenes, polyhedral boranes, and related structures. Chem. Rev. 2005, 105, 3613–3642. [Google Scholar] [CrossRef] [PubMed]
- Poater, J.; Sola, M.; Viñas, C.; Teixidor, F. π Aromaticity and three-dimensional aromaticity: Two sides of the same coin? Angew. Chem. Int. Ed. 2014, 53, 12191–12195. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J. Challenges and opportunities for the application of boron clusters in drug design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Ďorďovič, V.; Tošner, Z.; Uchman, M.; Zhigunov, A.; Reza, M.; Ruokolainen, J.; Pramanik, G.; Cígler, P.; Kalíková, K.; Gradzielski, M.; et al. Stealth Amphiphiles: Self-Assembly of polyhedral boron clusters. Langmuir 2016, 32, 6713–6722. [Google Scholar] [CrossRef]
- Bauduin, P.; Prevost, S.; Farrs, P.; Teixidor, F.; Diat, O.; Zemb, T. A theta-shaped amphiphilic cobaltabisdicarbollide anion: Transition from monolayer vesicles to micelles. Angew. Chem. Int. Ed. 2011, 50, 5298–5300. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Sivaev, I.B.; Dubey, R.D.; Semioshkin, A.; Shmal’ko, A.V.; Kosenko, I.D.; Lebedeva, K.V.; Mandal, S.; Sreejyothi, P.; Sarkar, A.; et al. Boron-containing lipids and liposomes: New conjugates of cholesterol with polyhedral boron hydrides. Chem. Eur. J. 2020, 26, 13832–13841. [Google Scholar] [CrossRef]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sarosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-Containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [Green Version]
- Schaffran, T.; Li, J.Y.; Karlsson, G.; Edwards, K.; Winterhalter, M.; Gabel, D. Interaction of N,N,N-trialkylammonioundecahydro-closo-dodecaborates with dipalmitoyl phosphatidylcholine liposomes. Chem. Phys. Lipids. 2010, 163, 64–73. [Google Scholar] [CrossRef]
- Assaf, K.I.; Begaj, B.; Frank, A.; Nilam, M.; Mougharbel, A.S.; Kortz, U.; Nekvinda, J.; Grüner, B.; Gabel, D.; Nau, W.M. High-affinity binding of metallacarborane cobalt bis(dicarbollide) anions to cyclodextrins and application to membrane translocation. J. Org. Chem. 2019, 84, 11790–11798. [Google Scholar] [CrossRef] [PubMed]
- Verdiá-Báguena, C.; Alcaraz, A.; Aguilella, V.M.; Cioran, A.M.; Tachikawa, S.; Nakamura, H.; Teixidor, F.; Viñas, C. Amphiphilic COSAN and I2-COSAN crossing synthetic lipid membranes: Planar bilayers and liposomes. Chem. Comm. 2014, 50, 6700–6703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarrés, M.; Canetta, E.; Paul, E.; Forbes, J.; Azzouni, K.; Viñas, C.; Teixidor, F.; Harwood, A. Biological interaction of COSAN-based synthetic vesicles with living cells. J. Sci. Rep. 2015, 5, 7804–7811. [Google Scholar] [CrossRef]
- Grüner, B.; Brynda, J.; Das, V.; Šícha, V.; Štěpánková, J.; Nekvinda, J.; Holub, J.; Pospíšilová, K.; Fábry, M.; Pachl, P.; et al. Metallacarborane sulfamides: Unconventional, specific, and highly selective inhibitors of carbonic anhydrase. J. Med. Chem. 2019, 62, 9560–9575. [Google Scholar] [CrossRef]
- Pecina, A.; Lepsik, M.; Řezáč, J.; Brynda, J.; Mader, P.; Řezáčová, P.; Hobza, P.; Fanfrlik, J. QM/MM calculations reveal the different nature of the interaction of two carborane-based sulfamide inhibitors of human carbonic anhydrase II. J. Phys. Chem. 2013, 117, 16096–16104. [Google Scholar] [CrossRef] [PubMed]
- Sivaev, B.I.; Bregadze, V.I. Polyhedral boranes for medical applications: Current status and perspectives. Eur. J. Inorg. Chem. 2009, 11, 1433–1450. [Google Scholar] [CrossRef]
- Fayaz, A.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef] [Green Version]
- Goswami, L.N.; Ma, L.; Chakravarty, S.; Cai, Q.; Jalisatgi, S.S.; Hawthorne, M.F. Discrete nanomolecular polyhedral borane scaffold supporting multiple gadolinium(III) complexes as a high performance MRI contrast agent. Inorg. Chem. 2013, 52, 1694–1700. [Google Scholar] [CrossRef]
- Ilinova, A.; Semioshkin, A.; Lobanova, I.; Bregadze, V.I.; Mironov, A.F.; Paradowska, E.; Studzińska, M.; Jabłońska, A.; Białek-Pietras, M.; Leśnikowski, Z.J. Synthesis, cytotoxicity and antiviral activity studies of the conjugates of cobalt bis (1,2-dicarbollide)(-I) with 5-ethynyl-2′-deoxyuridine and its cyclic derivatives. Tetrahedron 2014, 70, 5704–5710. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically active compounds based on membranotropic cage carriers-derivatives of adamantane and polyhedral boron clusters (Review). Russ. J. Inorg. Chem. 2022, 67, 28–47. [Google Scholar] [CrossRef]
- Fink, K.; Uchman, M. Boron cluster compounds as new chemical leads for antimicrobial Therapy. Coord. Chem. Rev. 2021, 431, 213684–213693. [Google Scholar] [CrossRef]
- Plesek, J. Potential Applications of the Boron Cluster Compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- Totani, T.; Aono, K.; Yamamoto, K.; Tawara, K. Synthesis and in vitro antimicrobial property of o-carborane derivatives. J. Med. Chem. 1981, 24, 1492–1499. [Google Scholar] [CrossRef]
- Popova, T.; Zaulet, A.; Teixidor, F.; Alexandrova, R.; Vinas, C. Investigations on antimicrobial activity of cobaltabisdicarbollides. J. Organomet. Chem. 2013, 747, 229–234. [Google Scholar] [CrossRef]
- Swietnicki, W.; Goldeman, W.; Psurski, M.; Nasulewicz-Goldeman, A.; Boguszewska-Czubara, A.; Drab, M.; Sycz, J.; Goszczyński, T.M. Metallacarborane derivatives effective against Pseudomonas aeruginosa and Yersinia enterocolitica. Int. J. Mol. Sci. 2021, 22, 6762. [Google Scholar] [CrossRef]
- Zheng, Y.K.; Liu, W.W.; Chen, Y.; Jiang, H.; Yan, H.; Kosenko, I.; Chekulaeva, L.; Sivaev, I.; Bregadze, V.; Wang, X.M. A highly potent antibacterial agent targeting methicillin-resistant staphylococcus aureus based on cobalt bis (1,2-dicarbollide) alkoxy derivative. Organometallics 2017, 36, 3484–3490. [Google Scholar] [CrossRef]
- Kvasnickova, E.; Masak, J.; Cejka, J.; Matatkova, O.; Sicha, V. Preparation, characterization, and the selective antimicrobial activity of N-alkylammonium 8-diethyleneglycol cobalt bis-dicarbollide derivatives. J. Organomet. Chem. 2017, 827, 23–31. [Google Scholar] [CrossRef]
- Vankova, E.; Lokocova, K.; Matatkova, O.; Krizova, I.; Masak, J.; Grüner, B.; Kaule, P.; Cermak, J.; Sicha, V. Cobalt bis-dicarbollide and its ammonium derivatives are effective antimicrobial and antibiofilm agents. J. Organomet. Chem. 2019, 899, 120891–120899. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Yang, G.; Byun, S.; Rao, G.; Shoen, C.; Yang, H.; Gulati, A.; Crick, D.C.; Cynamon, M.; et al. Oxa, thia, heterocycle, and carborane analogues of SQ109: Bacterial and protozoal cell growth inhibitors. ACS Infect. Dis. 2015, 1, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Leśnikowski, Z.J.; Schinazi, R.F. Carboranyl oligonucleotides. Synthesis of thymidine (3′,5′) thymidine (o-carboran-1-ylmethy1) phosphonate. Org. Chem. 1993, 58, 6531–6535. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J.; Shi, J.; Schinazi, R.F. Nucleic acids and nucleosides containing carboranes. J. Organomet. Chem. 1999, 581, 156–169. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhang, J.L.; Zhang, Y.B.; Liu, J.Y.; Veen, S.; Duttwyler, S. The closo-dodecaborate dianion fused with oxazoles provides 3D diboraheterocycles with selective antimicrobial activity. Chemistry 2018, 24, 10364–10371. [Google Scholar] [CrossRef] [PubMed]
- Oros, G.; Ujvary, I.; Nachman, R.J. Antimicrobial activity of o-carboranylalanine. Amino Acids. 1999, 17, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wu, C.Y.; Lv, X.Y.; Tang, X.; Zhao, X.Q.; Yan, H.; Jiang, H.; Wang, X.M. Discovery of ferrocene-carborane derivatives as novel chemical antimicrobial agents against multidrug-resistant bacteria. Sci. China Chem. 2012, 55, 2388–2395. [Google Scholar] [CrossRef]
- Li, S.; Wu, C.; Zhao, X.; Jiang, H.; Yan, H.; Wang, X. Synergistic Antibacterial Activity of New Isomeric Carborane Derivatives Through Combination with Nanoscaled Titania. J. Biomed. Nanotechnol. 2013, 9, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wang, Z.J.; Wei, Y.F.; Wu, C.Y.; Gao, S.P.; Jiang, H.; Zhao, X.Q.; Yan, H.; Wang, X.M. Antimicrobial activity of a ferrocene-substituted carborane derivative targeting multidrug-resistant infection. Biomaterials 2013, 34, 902–911. [Google Scholar] [CrossRef]
- Adamska, A.; Rumijowska-Galewicz, A.; Ruszczynska, A.; Studzińska, M.; Jabłońska, A.; Paradowska, E.; Bulska, E.; Munier-Lehmann, H.; Dziadek, J.; Leśnikowski, Z.J.; et al. Anti-mycobacterial activity of thymine derivatives bearing boron clusters. Eur. J. Med. Chem. 2016, 121, 71–81. [Google Scholar] [CrossRef]
- Rózycka, D.; Leśnikowski, Z.J.; Olejniczak, A.B. Synthesis of boron cluster analogs of penicillin and their antibacterial activity. J. Organomet. Chem. 2019, 881, 19–24. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin-from molecule to biological function. Angew. Chem. Int. Ed. Eng. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res. Int. 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaeelzadeh, M.; Salehi, P.; Bararjanian, M.; Gharaghani, S. Synthesis of new triazole tethered derivatives of curcumin and their antibacterial and antifungal properties. J. Iran. Chem. Soc. 2019, 16, 465–477. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Rani, A.; Singh, G.; Singh, A.; Maqbool, U.; Kaur, G.; Singh, J. CuAAC-ensembled 1,2,3-triazole-linked isosteres as pharmacophores in drug discovery: Review. RSC Adv. 2020, 10, 5610–5635. [Google Scholar] [CrossRef]
- Smyshliaeva, L.A.; Varaksin, M.V.; Fomina, E.I.; Joy, M.N.; Bakulev, V.A.; Charushin, V.N.; Chupakhin, O.N. Cu(I)-catalyzed cycloaddition of vinylacetylene ortho-carborane and arylazides in the design of 1,2,3-triazolyl-modified vinylcarborane fluorophores. Organometalics 2020, 39, 3679–3688. [Google Scholar] [CrossRef]
- Wojtczak, B.A.; Andrysiak, A.; Grüner, B.; Leśnikowski, Z.J. “Chemical ligation”: A versatile method for nucleoside modification with boron clusters. Chem. Eur. J. 2008, 14, 10675–10682. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Semioshkin, A.A.; Las´kova, J.N.; Berzina, M.Y.; Lobanova, I.A.; Sivaev, I.B.; Grin, M.A.; Titeev, R.A.; Brittal, D.I.; Ulybina, O.V.; et al. Novel types of boronated chlorine e6 conjugates via «click chemistry». Appl. Organometal. Chem. 2009, 23, 370–374. [Google Scholar] [CrossRef]
- Dubey, R.D.; Sarkar, A.; Shen, Z.; Bregadze, V.I.; Sivaev, I.B.; Druzina, A.A.; Zhidkova, O.B.; Shmal’ko, A.V.; Kosenko, I.D.; Sreejyothi, P.; et al. Effects of linkers on the development of liposomal formulation of cholesterol conjugated cobalt bis (dicarbollides). J. Pharm. Sci. 2020, 110, 1365–1373. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Andreichuk, E.P.; Zhidkova, O.B.; Kosenko, I.D.; Semioshkin, A.A.; Sivaev, I.B.; Mandal, S.; Shen, Z.; Bregadze, V.I. “Click” synthesis of cobalt bis (dicarbollide)-cholesterol conjugates. Mendeleev Commun. 2019, 29, 628–630. [Google Scholar] [CrossRef]
- Druzina, A.A.; Kosenko, I.D.; Zhidkova, O.B.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Novel cobalt bis (dicarbollide) based on terminal alkynes and their “click” reactions. Eur. J. Inorg. Chem. 2020, 27, 2658–2665. [Google Scholar] [CrossRef]
- Druzina, A.A.; Shmalko, A.V.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of cobalt and iron bis (dicarbollide)s and their use in organic synthesis. Russ. Chem. Rev. 2021, 90, 785–830. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Kosenko, I.D. Synthesis of conjugates of closo-dodecaborate dianion with cholesterol using a “click” reaction. Russ. Chem. Bull. Int. Ed. 2020, 69, 1080–1084. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Kosenko, I.D.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Synthesis and structure of nido-carboranyl azide and its “click” reactions. Molecules 2021, 26, 530. [Google Scholar] [CrossRef]
- Druzina, A.A.; Stogniy, M.Y. Synthesis of cholesterol derivatives based on closo- and nido-carboranes. Chem. Bull. Int. Ed. 2021, 70, 527–532. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Nekrasova, N.A.; Suponitsky, K.Y.; Timofeev, S.V.; Bregadze, V.I. Synthesis of zwitter-ionic conjugate of nido-carborane with cholesterol. Molecules 2021, 26, 6687. [Google Scholar] [CrossRef]
- Alberti, D.; Protti, N.; Franck, M.; Stefania, R.; Bortolussi, S.; Altieri, S.; Deagostino, A.; Aime, S.; Crich, S.G. “Theranostic” nanoparticles loaded with imaging probes and rubrocurcumin for a combined cancer therapy by folate receptor targeting. ChemMedChem 2017, 12, 502–507. [Google Scholar] [CrossRef]
- Kosenko, I.D.; Lobanova, I.A.; Chekulaeva, L.A.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 1,4-disubstituted 1,2,3-triazoles based on cobalt bis (1,2-dicarbollide). Russ. Chem. Bull. Int. Ed. 2013, 62, 497–503. [Google Scholar] [CrossRef]
- Orlova, A.V.; Kondakov, N.N.; Kimel, B.G.; Kononov, L.O.; Kononova, E.G.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel derivatives of closo-dodecaborate anion with azido group at the terminal position of the spacer. Appl. Organomet. Chem. 2007, 21, 98–100. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Osipov, S.N.; Grebenyuk, J.N.; Nizhnik, E.A.; Godovikov, I.A.; Shchetnikov, G.T.; Bregadze, V.I. An Effective Approach to 1,2,3-triazole Containing 12-vertex closo-dodecaborates. Collect. Czech. Chem. Commun. 2007, 72, 1717–1724. [Google Scholar] [CrossRef]
- Averick, A.; Dolai, S.; Punia, A.; Punia, K.; Guarigli, S.R.; L’Amoreaux, W.; Hong, K.; Raja, K. Green anchors: Chelating properties of ATRP-click curcumin-polymer conjugates. React. Funct. Polym. 2016, 102, 47–52. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI) 2015 (M07-A10). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 10th ed.; Approved Standard; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Clinical and Laboratory Standards Institute M38-A2 CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved Standard; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI document M38-A, Antifungal Susceptibility Testing of Yeasts: M27-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.


| Organism | MIC (mg/L) | |||||
|---|---|---|---|---|---|---|
| Curcumin | 4 | 5 | 9 | 10 | 11 | |
| Gram-negative bacteria | ||||||
| Escherichia coli ATCC 25922 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Pseudomonas aeruginosa ATCC 27853 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Acinetobacter baumannii 73 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Gram-positive bacteria | ||||||
| Staphylococcus aureus ATCC 29213 | 62.5 | 1000 | 31.25 | >1000 | >1000 | >1000 |
| Staphylococcus aureus (MRSA) 17 | 125 | 1000 | 31.25 | >1000 | >1000 | >1000 |
| Bacillus cereus ATCC 10702 | 125 | 500 | 62.5 | 500 | 250 | 250 |
| Enterococcus faecalis ATCC 29212 | >1000 | >1000 | 250 | >1000 | >1000 | >1000 |
| Fungal streins | ||||||
| Candida albicans 604M | >1000 | >1000 | >100 | >1000 | >1000 | >1000 |
| Candida albicans 8P | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Aspergillus fumigatus ATCC 46645 | >1000 | >1000 | 125 | >1000 | >1000 | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druzina, A.A.; Grammatikova, N.E.; Zhidkova, O.B.; Nekrasova, N.A.; Dudarova, N.V.; Kosenko, I.D.; Grin, M.A.; Bregadze, V.I. Synthesis and Antibacterial Activity Studies of the Conjugates of Curcumin with closo-Dodecaborate and Cobalt Bis(Dicarbollide) Boron Clusters. Molecules 2022, 27, 2920. https://doi.org/10.3390/molecules27092920
Druzina AA, Grammatikova NE, Zhidkova OB, Nekrasova NA, Dudarova NV, Kosenko ID, Grin MA, Bregadze VI. Synthesis and Antibacterial Activity Studies of the Conjugates of Curcumin with closo-Dodecaborate and Cobalt Bis(Dicarbollide) Boron Clusters. Molecules. 2022; 27(9):2920. https://doi.org/10.3390/molecules27092920
Chicago/Turabian StyleDruzina, Anna A., Natalia E. Grammatikova, Olga B. Zhidkova, Natalia A. Nekrasova, Nadezhda V. Dudarova, Irina D. Kosenko, Mikhail A. Grin, and Vladimir I. Bregadze. 2022. "Synthesis and Antibacterial Activity Studies of the Conjugates of Curcumin with closo-Dodecaborate and Cobalt Bis(Dicarbollide) Boron Clusters" Molecules 27, no. 9: 2920. https://doi.org/10.3390/molecules27092920