Phytochemical Composition and Protective Effect of Vernonanthura polyanthes Leaf against In Vivo Doxorubicin-Mediated Toxicity
Abstract
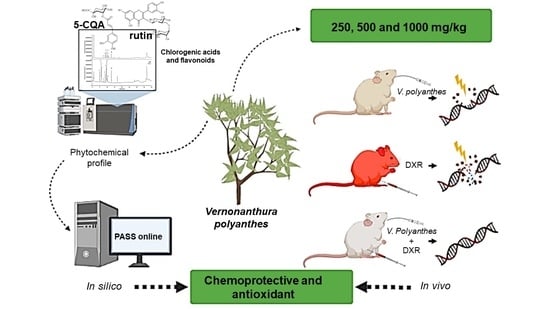
:1. Introduction
2. Methodology
2.1. Botanical Material
2.2. Infusion Preparation and Fractionation
2.3. Animals
2.4. In Vivo Experimental Procedures
2.5. Micronucleus Test (MN)
2.6. Comet Assay
2.7. Statistical Analysis
2.8. Chemical Profiles
2.9. Prediction of Activity Spectra for Substances
3. Results
3.1. Cytogenotoxic Evaluation
3.2. Anticytogenotoxic Evaluation
3.3. Chemical Profiles of VpLAE and n-BF
3.4. Biological Activity Prediction of Identified Secondary Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V. Phytochemical and biological studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef]
- Majeed, I.; Rizwan, K.; Ashar, A.; Rasheed, T.; Amarowicz, R.; Kausar, H.; Zia-ul-haq, M.; Marceanu, L.G. A comprehensive review of the ethnotraditional uses and biological and pharmacological potential of the genus mimosa. Int. J. Mol. Sci. 2021, 22, 7463. [Google Scholar] [CrossRef]
- Bailão, E.F.L.C.; Devilla, I.A.; da Conceição, E.C.; Borges, L.L. Bioactive compounds found in Brazilian cerrado fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, H.; Matos, F.J. Plantas Medicinais no Brasil. Nativas e Exóticas, 2nd ed.; Instituto, Plantarum: Nova Odessa, Brazil, 2008; ISBN 978-8586714283. [Google Scholar]
- Rodrigues, V.E.G.; Carvalho, D.A. De Levantamento Etnobotânico De Plantas Medicinais No Domíniodo Cerrado Na Região Do Alto Etnobotanical Survey of Medicinal Plants in the Dominion of Meadows in the Region of the Alto Rio Grande—Minas Gerais. Ciênc. Agratecnol. 2001, 25, 102–123. [Google Scholar]
- Silveira, R.R.; Foglio, M.A.; Gontijo, J.A.R. Effect of the crude extract of Vernonia polyanthes Less. on blood pressure and renal sodium excretion in unanesthetized rats. Phytomedicine 2003, 10, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Braga, F.G.; Bouzada, M.L.M.; Fabri, R.L.; de O. Matos, M.; Moreira, F.O.; Scio, E.; Coimbra, E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007, 111, 396–402. [Google Scholar] [CrossRef]
- Barbastefano, V.; Cola, M.; Luiz-Ferreira, A.; Farias-Silva, E.; Hiruma-Lima, C.A.; Rinaldo, D.; Vilegas, W.; Souza-Brito, A.R.M. Vernonia polyanthes as a new source of antiulcer drugs. Fitoterapia 2007, 78, 545–551. [Google Scholar] [CrossRef]
- dos Santos Temponi, V.; Da Silva, J.B.; Alves, M.S.; Ribeiro, A.; Pinho, J.D.; Yamamoto, C.H.; Pinto, M.A.; Del-Vechio-Vieira, G.; De Sousa, O.V. Antinociceptive and anti-inflammatory effects of ethanol extract from Vernonia polyanthes leaves in rodents. Int. J. Mol. Sci. 2012, 13, 3887–3899. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.G.; Prince, K.A.; Higuchi, C.T.; Santos, A.C.B.; Lopes, L.M.X.; Simões, M.J.S.; Leite, C.Q.F. Antimycobacterial activity of some Brazilian indigenous medicinal drinks. Rev. Cienc. Farm. Basica E Aplicada 2007, 28, 165–169. [Google Scholar]
- Silva, N.C.C.; Barbosa, L.; Seito, L.N.; Junior, A.F. Antimicrobial activity and phytochemical analysis of crude extracts and essential oils from medicinal plants. Nat. Prod. Res. 2012, 26, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Formulário de Fitoterápicos da Farmacopeia Brasileira; 2011 Brasília-DF, Brazil. pp. 1–126. Available online: https://www.gov.br/anvisa/pt-br/assuntos/farmacopeia/formulario-fitoterapico/arquivos/2021-fffb2-final-c-capa2.pdf (accessed on 1 March 2022).
- Almeida, L.M.; Prado, A.D.L.; Xavier-Silva, K.R.; Firmino, M.T.; Paula, M.I.M.; Gomes, P.N.; Paula, J.A.M.; Bailão, E.F.L.C. Cytotoxic effect of Vernonanthura polyanthes leaves aqueous extracts. Braz. J. Biol. 2021, 81, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Martucci, M.E.P.; De Vos, R.C.H.; Carollo, C.A.; Gobbo-neto, L. Metabolomics as a Potential Chemotaxonomical Tool: Application in the Genus Vernonia Schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef] [Green Version]
- Feleti, S.M.V.; Aleluia, R.L.; Gervásio, S.V.; Dutra, J.C.V.; Oliveira, J.R.P.; Gonçalves, R.C.R.; Jamal, C.M.; Kuster, R.M.; Brasileiro, B.G.; Battituci, M.C.R. Phytochemical screening, antioxidant, anti-cytotoxic and anticancer effects of Galinsoga parviflora and Vernonia polyanthes (asteraceae) extracts. Int. J. Res. Granthaalayah 2020, 8, 84–98. [Google Scholar] [CrossRef]
- Gallon, M.E.; Jaiyesimi, O.A.; Gobbo-Neto, L. LC-UV-HRMS dereplication of secondary metabolites from Brazilian Vernonieae (Asteraceae) species supported through in-house database. Biochem. Syst. Ecol. 2018, 78, 5–16. [Google Scholar] [CrossRef]
- Igual, M.O.; Martucci, M.E.P.; Da Costa, F.B.; Gobbo-Neto, L. Sesquiterpene lactones, chlorogenic acids and flavonoids from leaves of Vernonia polyanthes Less (Asteraceae). Biochem. Syst. Ecol. 2013, 51, 94–97. [Google Scholar] [CrossRef]
- Guerra-Santos, I.J.; Rocha, J.D.; Vale, C.R.; Sousa, W.C.; Teles, A.M.; Chen-Chen, L.; Carvalho, S.; Bailão, E.F.L.C. Vernonanthura polyanthes leaves aqueous extract enhances doxorubicin genotoxicity in somatic cells of Drosophila melanogaster and presents no antifungal activity against Candida spp. Braz. J. Biol. 2016, 76, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgetto, G.V.; Boriolo, M.F.; Silva, L.M.; Nogueira, D.A.; da Silva José, T.D.; Ribeiro, G.E.; Oliveira, N.D.; Fiorini, J.E. Ensaios de atividade antimicrobiana in vitro e mutagênica in vivo com extrato de Vernonia polyanthes Less (Assa-peixe). Rev. Do Inst. Adolfo Lutz 2011, 70, 53–61. [Google Scholar]
- Rocha, J.D.; da Silva Ferreira, J.; Vieira Silva, J.G.; Silva Fernandes, A.; Hollanda Véras, J.; Madureira de Almeida, L.; Magalhães Teles, A.; Luiz Borges, L.; Chen-Chen, L.; Luiz Cardoso Bailão, E.F. In vitro hematotoxicity of Vernonanthura polyanthes leaf aqueous extract and its fractions. Drug Chem. Toxicol. 2020, 1–9. [Google Scholar] [CrossRef]
- Xu, M.F.; Tang, P.L.; Qian, Z.M.; Ashraf, M. Effects by doxorubicin on the myocardium are mediated by oxygen free radicals. Life Sci. 2001, 68, 889–901. [Google Scholar] [CrossRef]
- Korga, A.; Józefczyk, A.; Zgórka, G.; Homa, M.; Ostrowska, M.; Burdan, F.; Dudka, J. Evaluation of the phytochemical composition and protective activities of methanolic extracts of Centaurea borysthenica and Centaurea daghestanica (Lipsky) wagenitz on cardiomyocytes treated with doxorubicin. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. New advances in molecular mechanisms and the prevention of adriamycin toxicity by antioxidant nutrients. Food Chem. Toxicol. 2010, 48, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Li, T.; Kumar, D.; Danelisen, I.; Iliskovic, N. Adriamycin-induced heart failure: Mechanism and modulation. Mol. Cell. Biochem. 2000, 207, 77–86. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 474: Mammalian Erythrocyte Micronucleus Test; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2016; p. 21. [Google Scholar] [CrossRef] [Green Version]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the pass online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Goel, R.K.; Singh, D.; Lagunin, A.; Poroikov, V. PASS-assisted exploration of new therapeutic potential of natural products. Med. Chem. Res. 2011, 20, 1509–1514. [Google Scholar] [CrossRef]
- Hayashi, M. The micronucleus test-most widely used in vivo genotoxicity test. Genes Environ. 2016, 38, 4–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heddle, J.A. A rapid in vivo test for chromosomal damage. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1973, 18, 187–190. [Google Scholar] [CrossRef]
- Collins, A.R.; Dušinská, M.; Horská, A. Detection of alkylation damage in human lymphocyte DNA with the comet assay. Acta Biochim. Pol. 2001, 48, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Appl. Biochem. Biotechnol. Part B Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Collins, A.R. Investigating oxidative DNA damage and its repair using the comet assay. Mutat. Res. Rev. Mutat. Res. 2009, 681, 24–32. [Google Scholar] [CrossRef]
- Hartmann, A.; Agurell, E.; Beevers, C.; Brendler-Schwaab, S.; Burlinson, B.; Clay, P.; Collins, A.; Smith, A.; Speit, G.; Thybaud, V.; et al. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis 2003, 18, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgorashi, E.E.; Taylor, J.L.S.; Maes, A.; De Kimpe, N.; Van Staden, J.; Verschaeve, L. The use of plants in traditional medicine: Potential genotoxic risks. S. Afr. J. Bot. 2002, 68, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Boeira, J.M.; Da Silva, J.; Erdtmann, B.; Henriques, J.A.P. Genotoxic effects of the alkaloids harman and harmine assessed by Comet assay and chromosome aberration test in mammalian cells in vitro. Pharmacol. Toxicol. 2001, 89, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Padial, C.; Puerto, M.; Prieto, A.I.; Ayala, N.; Beaumont, P.; Rouger, C.; Krisa, S.; Pichardo, S. In vivo genotoxicity evaluation of a stilbene extract prior to its use as a natural additive: A combination of the micronucleus test and the comet assay. Foods 2021, 10, 439. [Google Scholar] [CrossRef]
- Karkossa, I.; Bannuscher, A.; Hellack, B.; Wohlleben, W.; Laloy, J.; Stan, M.S.; Dinischiotu, A.; Wiemann, M.; Luch, A.; Haase, A.; et al. Nanomaterials induce different levels of oxidative stress, depending on the used model system: Comparison of in vitro and in vivo effects. Sci. Total Environ. 2021, 801, 149538. [Google Scholar] [CrossRef] [PubMed]
- Li, N. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 2002, 14, 459–486. [Google Scholar] [CrossRef]
- Brandão, F.; Costa, C.; Bessa, M.J.; Dumortier, E.; Debacq-Chainiaux, F.; Hubaux, R.; Salmon, M.; Laloy, J.; Stan, M.S.; Hermenean, A.; et al. Genotoxicity and gene expression in the rat lung tissue following instillation and inhalation of different variants of amorphous silica nanomaterials (Asio2 nm). Nanomaterials 2021, 11, 1502. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.; Kong, X.; Ding, X.; Gu, H.; Chu, T.; Ying, W. NAD+ administration decreases doxorubicin-induced liver damage of mice by enhancing antioxidation capacity and decreasing DNA damage. Chem. Biol. Interact. 2014, 212, 65–71. [Google Scholar] [CrossRef]
- Quiles, J.L.; Huertas, J.R.; Battino, M.; Mataix, J.; Ramírez-Tortosa, M.C. Antioxidant nutrients and adriamycin toxicity. Toxicology 2002, 180, 79–95. [Google Scholar] [CrossRef]
- Hall, S.; Anoopkumar-Dukie, S.; Grant, G.D.; Desbrow, B.; Lai, R.; Arora, D.; Hong, Y. Modulation of chemotherapy-induced cytotoxicity in SH-SY5Y neuroblastoma cells by caffeine and chlorogenic acid. Toxicol. Mech. Methods 2017, 27, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.S.; Naveed, S.; Ahmed, A.; Abbas, Z.; Gull, I.; Athar, M.A. Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J. Cancer Ther. 2014, 05, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Dewanjee, S.; Joardar, S.; Bhattacharjee, N.; Dua, T.K.; Das, S.; Kalita, J.; Manna, P. Edible leaf extract of Ipomoea aquatica Forssk. (Convolvulaceae) attenuates doxorubicin-induced liver injury via inhibiting oxidative impairment, MAPK activation and intrinsic pathway of apoptosis. Food Chem. Toxicol. 2017, 105, 322–336. [Google Scholar] [CrossRef]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic acid enhances doxorubicin-mediated cytotoxic effect in osteosarcoma cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic compound contents and antioxidant activity in plants with nutritional and/or medicinal properties from the Peruvian Andean region. Ind. Crops Prod. 2013, 47, 145–152. [Google Scholar] [CrossRef]
- Shah, S.R.; Ukaegbu, C.I.; Hamid, H.A.; Alara, O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018, 12, 1947–1961. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Abdul Mudalip, S.K.; Olalere, O.A. Effect of drying methods on the free radicals scavenging activity of Vernonia amygdalina growing in Malaysia. J. King Saud Univ. Sci. 2017, 31, 495–499. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds, antioxidants, and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crops Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- Arantes, A.A.; Falé, P.L.; Costa, L.C.B.; Pacheco, R.; Ascensão, L.; Serralheiro, M.L. Inhibition of HMG-CoA reductase activity and cholesterol permeation through Caco-2 cells by caffeoylquinic acids from Vernonia condensata leaves. Rev. Bras. Farmacogn. 2016, 26, 738–743. [Google Scholar] [CrossRef]
- Amuthan, A.; Devi, V.; Shreedhara, C.S.; Rao, V.; Jasphin, S.; Kumar, N. Vernonia cinerea regenerates tubular epithelial cells in cisplatin induced nephrotoxicity in cancer bearing mice without affecting antitumor activity. J. Tradit. Complement. Med. 2021, 11, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; De Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Mara Serpeloni, J.; Mazzaron Barcelos, G.R.; Prates Mori, M.; Yanagui, K.; Vilegas, W.; Aparecida Varanda, E.; de Syllos Cólus, I.M. Cytotoxic and mutagenic evaluation of extracts from plant species of the Miconia genus and their influence on doxorubicin-induced mutagenicity: An in vitro analysis. Exp. Toxicol. Pathol. 2011, 63, 499–504. [Google Scholar] [CrossRef]
- Zhang, D.D. mechanistic studies of the NRF2-KEAP1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Reichard, J.F.; Motz, G.T.; Puga, A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007, 35, 7074–7086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.U.; Ahmed, O.M.; Fahim, H.I.; Ahmed, N.A.; Ashour, M.B. Effects of Rutin and Quercetin on Doxorubicin-Induced Renocardiotoxicity in Male Wistar Rats. Adv. Anim. Vet. Sci. 2020, 8, 370–384. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Yang, L.; Ma, J.; Lu, L.; Wang, X.; Ren, J.; Yang, J. Rutin attenuates doxorubicin-induced cardiotoxicity via regulating autophagy and apoptosis. BBA Mol. Basis Dis. 2016, 17, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T. Review Xenobiotic-Metabolizing Enzymes Involved in Activation and Detoxication of Carcinogenic Polycyclic Aromatic Hydrocarbons. Drug Metab. Pharmacokinet. 2006, 21, 257–276. [Google Scholar] [CrossRef] [Green Version]
- Rendic, S.P.; Guengerich, F.P. Human Family 1–4 Cytochrome P450 Enzymes Involved in the Metabolic Activation of Xenobiotic and Physiological Chemicals: An Update; Springer: Berlin/Heidelberg, Germany, 2021; Volume 95, ISBN 0123456789. [Google Scholar]
- Chen, Z.; Xie, J.; Li, Q.; Hu, K.; Yang, Z.; Yu, H.; Liu, Y. Human CYP enzyme-activated clastogenicity of 2-ethylhexyl diphenyl phosphate (a flame retardant) in mammalian cells. Environ. Pollut. 2021, 285, 117527. [Google Scholar] [CrossRef] [PubMed]








| Animal Groups | Dose | Number of Animals (n) | Exposure Time |
|---|---|---|---|
| Controls | |||
| G1 | NC (H2O) | 5 | 24 h |
| G2 | PC (DXR 50 mg/kg ip) | 5 | 24 h |
| Genotoxicity | |||
| VpLAE | |||
| G3 | 250 mg/kg | 5 | 24 h |
| G4 | 500 mg/kg | 5 | 24 h |
| G5 | 1000 mg/kg | 5 | 24 h |
| G6 | 1000 mg/kg | 5 | 120 h |
| n-BF | |||
| G7 | 250 mg/kg | 5 | 24 h |
| G8 | 500 mg/kg | 5 | 24 h |
| G9 | 1000 mg/kg | 5 | 24 h |
| G10 | 1000 mg/kg | 5 | 120 h |
| Co-treatment | |||
| VpLAE | |||
| G11 | DXR + 250 mg/kg | 5 | 24 h |
| G12 | DXR + 500 mg/kg | 5 | 24 h |
| G13 | DXR +1000 mg/kg | 5 | 24 h |
| n-BF | |||
| G14 | DXR + 250 mg/kg | 5 | 24 h |
| G15 | DXR + 500 mg/kg | 5 | 24 h |
| G16 | DXR + 1000 mg/kg | 5 | 24 h |
| Pre-treatment | |||
| VpLAE | |||
| G17 | 250 mg/kg + DXR | 5 | 120 h |
| G18 | 500 mg/kg + DXR | 5 | 120 h |
| G19 | 1000 mg/kg + DXR | 5 | 120 h |
| n-BF | |||
| G20 | 250 mg/kg + DXR | 5 | 120 h |
| G21 | 500 mg/kg + DXR | 5 | 120 h |
| G22 | 250 mg/kg + DXR | 5 | 120 h |
| Post-treatment | |||
| VpLAE | |||
| G23 | DXR + 250 mg/kg | 5 | 24 h |
| G24 | DXR + 500 mg/kg | 5 | 24 h |
| G25 | DXR + 1000 mg/kg | 5 | 24 h |
| n-BF | |||
| G26 | DXR + 250 mg/kg | 5 | 24 h |
| G27 | DXR + 500 mg/kg | 5 | 24 h |
| G28 | DXR + 1000 mg/kg | 5 | 24 h |
| Metabolites | VpLAE | n-BF | ||
|---|---|---|---|---|
| O-caffeoylquinic acids | ||||
 |  |  | X | |
| 3-CQA | 4-CQA | 5-CQA | ||
| O-feruloylquinic acid | ||||
 | X | X | ||
| 5-FQA | ||||
| di-O-caffeoylquinic acids | ||||
 |  |  | X | X |
| 3,4-di-CQA | 3,5-di-CQA | 4,5-di-CQA | ||
| Flavonoid | ||||
 | X | X | ||
| rutin | ||||
| 3-CQA | 5-CQA | 4-CQA | 5-FQA | RUTIN | 3,4-di-CQA | 3,5-di-CQA | 4,5-di-CQA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological Activity | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi | Pa | Pi |
| Antimutagenic | N/A | N/A | N/A | N/A | 0.946 | 0.001 | 0.966 | 0.001 | N/A | N/A | 0.955 | 0.001 | 0.961 | 0.001 | 0.955 | 0.001 |
| Anticarcinogenic | 0.846 | 0.004 | 0.846 | 0.004 | 0.824 | 0.004 | 0.863 | 0.003 | 0.983 | 0.001 | 0.850 | 0.004 | 0.837 | 0.004 | 0.850 | 0.004 |
| Antineoplastic | 0.778 | 0.014 | 0.778 | 0.014 | 0.756 | 0.018 | 0.792 | 0.013 | 0.849 | 0.007 | 0.790 | 0.013 | 0.777 | 0.015 | 0.790 | 0.013 |
| Chemopreventive | 0.833 | 0.003 | 0.833 | 0.003 | 0.812 | 0.004 | 0.875 | 0.003 | 0.968 | 0.001 | 0.830 | 0.003 | 0.827 | 0.003 | 0.830 | 0.003 |
| Antioxidant | 0.785 | 0.004 | 0.833 | 0.003 | 0.771 | 0.004 | 0.727 | 0.004 | 0.923 | 0.003 | 0.806 | 0.003 | 0.780 | 0.004 | 0.806 | 0.003 |
| Free radical scavenger | 0.856 | 0.002 | 0.856 | 0.002 | 0.830 | 0.002 | 0.913 | 0.002 | 0.988 | 0.001 | 0.848 | 0.002 | 0.841 | 0.002 | 0.848 | 0.002 |
| Enzymatic Activity | 3-CQA | 5-CQA | 4-CQA | 5-FQA | RUTIN | 3,4-di-CQA | 3,5-di-CQA | 4,5-di-CQA |
|---|---|---|---|---|---|---|---|---|
| CYP1A inducer | N/A | N/A | N/A | N/A | 0.980 | N/A | N/A | N/A |
| CYP1A1 inducer | N/A | N/A | N/A | N/A | 0.970 | N/A | N/A | N/A |
| CYP3A4 inducer | N/A | N/A | N/A | N/A | 0.850 | N/A | N/A | N/A |
| CYP2C9 inducer | N/A | N/A | N/A | N/A | 0.830 | N/A | N/A | N/A |
| CYP3A inducer | N/A | N/A | N/A | N/A | 0.820 | N/A | N/A | N/A |
| CYP2H substrate | N/A | N/A | N/A | N/A | 0.820 | N/A | N/A | N/A |
| UDP-glucuronosyltransferase substrate | 0.710 | 0.710 | 0.820 | 0.850 | 0.970 | 0.830 | 0.840 | 0.830 |
| Aryl sulfotransferase inhibitor | N/A | N/A | 0.940 | 0.997 | N/A | 0.950 | 0.950 | 0.950 |
| Glutathione-disulfide reductase inhibitor | N/A | N/A | N/A | N/A | 0.740 | N/A | N/A | N/A |
| Lipid peroxidase inhibitor | 0.850 | 0.850 | 0.830 | 0.880 | 0.999 | 0.850 | 0.840 | 0.850 |
| HMOX1 expression enhancer | N/A | N/A | N/A | N/A | 0.750 | N/A | N/A | N/A |
| NADPH oxidase inhibitor | N/A | N/A | N/A | N/A | 0.850 | N/A | N/A | N/A |
| β-glucuronidase inhibitor | N/A | N/A | N/A | N/A | 0.760 | N/A | N/A | N/A |
| α-glucosidase inhibitor | N/A | N/A | N/A | N/A | 0.860 | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, J.D.; Gallon, M.E.; de Melo Bisneto, A.V.; Santana Amaral, V.C.; de Almeida, L.M.; Borges, L.L.; Chen-Chen, L.; Gobbo-Neto, L.; Bailão, E.F.L.C. Phytochemical Composition and Protective Effect of Vernonanthura polyanthes Leaf against In Vivo Doxorubicin-Mediated Toxicity. Molecules 2022, 27, 2553. https://doi.org/10.3390/molecules27082553
Rocha JD, Gallon ME, de Melo Bisneto AV, Santana Amaral VC, de Almeida LM, Borges LL, Chen-Chen L, Gobbo-Neto L, Bailão EFLC. Phytochemical Composition and Protective Effect of Vernonanthura polyanthes Leaf against In Vivo Doxorubicin-Mediated Toxicity. Molecules. 2022; 27(8):2553. https://doi.org/10.3390/molecules27082553
Chicago/Turabian StyleRocha, Jamira Dias, Marilia Elias Gallon, Abel Vieira de Melo Bisneto, Vanessa Cristiane Santana Amaral, Luciane Madureira de Almeida, Leonardo Luiz Borges, Lee Chen-Chen, Leonardo Gobbo-Neto, and Elisa Flávia Luiz Cardoso Bailão. 2022. "Phytochemical Composition and Protective Effect of Vernonanthura polyanthes Leaf against In Vivo Doxorubicin-Mediated Toxicity" Molecules 27, no. 8: 2553. https://doi.org/10.3390/molecules27082553