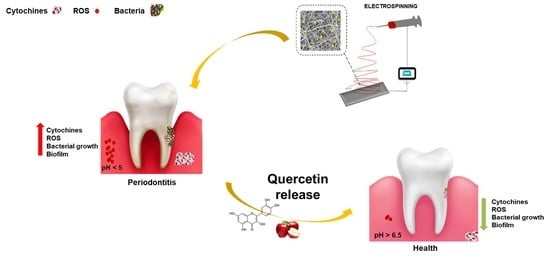
PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Electrospinning Solutions and Membrane Manufacturing
2.3. Characterization of Electrospun Membranes
2.4. Encapsulation Efficiency and In Vitro Drug Release Assay
2.5. Antioxidant Studies
2.6. Antibacterial Studies
2.6.1. Antimicrobial Activity
2.6.2. Biofilm Analysis
2.6.3. Quorum Sensing (QS) Interfering
2.7. In Vitro Cell Studies
2.7.1. Oxidative Stress and Lipid Peroxidation
2.7.2. Anti-Inflammatory Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Membrane Characterization
3.2. In Vitro Drug Release
3.3. Antimicrobial and Antibiofilm Activity
3.4. QUE-Loaded Membranes Inhibit LPS-Induced Antioxidant Activity
3.5. Anti-Inflammatory Effects of PLA-QUE on LPS-Stimulated HGFs
3.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tonetti, M.S.; Eickholz, P.; Loos, B.G.; Papapanou, P.; van der Velden, U.; Armitage, G.; Bouchard, P.; Deinzer, R.; Dietrich, T.; Hughes, F.; et al. Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Kononen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Demmer, R.T.; Papapanou, P.N. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol. 2000 2010, 53, 28–44. [Google Scholar] [CrossRef] [Green Version]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol. 2000 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. 2000 2020, 83, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Ogawa, H. Strengthening the prevention of periodontal disease: The WHO approach. J. Periodontol. 2005, 76, 2187–2193. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef] [PubMed]
- Taheri, J.B.; Azimi, S.; Rafieian, N.; Zanjani, H.A. Herbs in dentistry. Int. Dent. J. 2011, 61, 287–296. [Google Scholar] [CrossRef]
- Ara, T.; Nakatani, S.; Kobata, K.; Sogawa, N.; Sogawa, C. The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region. Medicines 2018, 5, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Elnagdy, S.; Raptopoulos, M.; Kormas, I.; Pedercini, A.; Wolff, L.F. Local Oral Delivery Agents with Anti-Biofilm Properties for the Treatment of Periodontitis and Peri-Implantitis. A Narrative Review. Molecules 2021, 26, 5661. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Fu, J.; Wu, W.; Ma, P.; Ren, L.; Yi, Z.; Wu, J. Quercetin Prevents Oxidative Stress-Induced Injury of Periodontal Ligament Cells and Alveolar Bone Loss in Periodontitis. Drug Des. Devel. 2021, 15, 3509–3522. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, L.; Zhao, B.; Xiong, Y.; Wang, Y.N.; Liang, J.; Xu, X. Quercetin reverses TNFalpha induced osteogenic damage to human periodontal ligament stem cells by suppressing the NFkappaB/NLRP3 inflammasome pathway. Int. J. Mol. Med. 2021, 47, 39. [Google Scholar] [CrossRef] [PubMed]
- Taskan, M.M.; Gevrek, F. Quercetin Decreased Alveolar Bone Loss and Apoptosis in Experimentally Induced Periodontitis Model in Wistar Rats. Antiinflamm. Antiallergy Agents Med. Chem. 2020, 19, 436–448. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, X.; Song, Z.; Li, L.; Chang, H.; Li, S.; Zhou, W. Quercetin inhibits virulence properties of Porphyromas gingivalis in periodontal disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef]
- Mooney, E.C.; Holden, S.E.; Xia, X.J.; Li, Y.; Jiang, M.; Banson, C.N.; Zhu, B.; Sahingur, S.E. Quercetin Preserves Oral Cavity Health by Mitigating Inflammation and Microbial Dysbiosis. Front. Immunol. 2021, 12, 774273. [Google Scholar] [CrossRef]
- Di Salle, A.; Viscusi, G.; Di Cristo, F.; Valentino, A.; Gorrasi, G.; Lamberti, E.; Vittoria, V.; Calarco, A.; Peluso, G. Antimicrobial and Antibiofilm Activity of Curcumin-Loaded Electrospun Nanofibers for the Prevention of the Biofilm-Associated Infections. Molecules 2021, 26, 4866. [Google Scholar] [CrossRef]
- Zhuang, Y.; Lin, K.; Yu, H. Advance of Nano-Composite Electrospun Fibers in Periodontal Regeneration. Front. Chem. 2019, 7, 495. [Google Scholar] [CrossRef]
- Budai-Szucs, M.; Ruggeri, M.; Faccendini, A.; Leber, A.; Rossi, S.; Varga, G.; Bonferoni, M.C.; Valyi, P.; Burian, K.; Csanyi, E.; et al. Electrospun Scaffolds in Periodontal Wound Healing. Polymers 2021, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Zhang, X.; Liu, N.; Yu, X.; Gao, M.; Wang, W.; Wu, T. Engineering Electrospun Nanofibers for the Treatment of Oral Diseases. Front. Chem. 2021, 9, 797523. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Bonadies, I.; Di Cristo, F.; Valentino, A.; Peluso, G.; Calarco, A.; Di Salle, A. pH-Responsive Resveratrol-Loaded Electrospun Membranes for the Prevention of Implant-Associated Infections. Nanomaterials 2020, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Georgieva, A.; Toshkova, R. Antioxidant and Antitumor Activities of Novel Quercetin-Loaded Electrospun Cellulose Acetate/Polyethylene Glycol Fibrous Materials. Antioxidants 2020, 9, 232. [Google Scholar] [CrossRef] [Green Version]
- Amrati, F.E.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evid. -Based Complement. Altern. Med. 2020, 2020, 8409718. [Google Scholar] [CrossRef] [Green Version]
- Mayer, S.; Tallawi, M.; De Luca, I.; Calarco, A.; Reinhardt, N.; Gray, L.A.; Drechsler, K.; Moeini, A.; Germann, N. Antimicrobial and physicochemical characterization of 2,3-dialdehyde cellulose-based wound dressings systems. Carbohydr. Polym. 2021, 272, 118506. [Google Scholar] [CrossRef]
- Tammaro, L.; Salle, A.D.; Calarco, A.; Luca, I.; Riccitiello, F.; Peluso, G.; Vittoria, V.; Sorrentino, A. Multifunctional Bioactive Resin for Dental Restorative Materials. Polymers 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kost, B.; Svyntkivska, M.; Brzeziński, M.; Makowski, T.; Piorkowska, E.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczyńska, A.; Biela, T. PLA/β-CD-based fibres loaded with quercetin as potential antibacterial dressing materials. Colloids Surf. B Biointerfaces 2020, 190, 110949. [Google Scholar] [CrossRef]
- Ajmal, G.; Bonde, G.V. Ciprofloxacin HCl and quercetin functionalized electrospun nanofiber membrane: Fabrication and its evaluation in full thickness wound healing. Artif. Cells Nanomed. Biotechnol. 2019, 47, 228–240. [Google Scholar] [CrossRef]
- Vashisth, P.; Singh, R.P.; Pruthi, V. A controlled release system for quercetin from biodegradable poly(lactide-co-glycolide)–polycaprolactone nanofibers and its in vitro antitumor activity. J. Bioact. Compat. Polym. 2015, 31, 260–272. [Google Scholar] [CrossRef]
- Bonadies, I.; Ambrogi, V.; Ascione, L.; Carfagna, C. A hyperbranched polyester as antinucleating agent for Artemisinin in electrospun nanofibers. Eur. Polym. J. 2014, 60, 145–152. [Google Scholar] [CrossRef]
- Peng, J.; Qian, Z.; Wang, B.; Fu, S.; Guo, G.; Luo, F.; Li, R.; Wu, D. Preparation and release characteristic of quercetin loaded poly(lactic acid) ultrafine fibers. J. Nanosci. Nanotechnol. 2011, 11, 3659–3668. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Nikhil, K.; Pemmaraju, S.C.; Pruthi, P.A.; Mallick, V.; Singh, H.; Patel, A.; Mishra, N.C.; Singh, R.P.; Pruthi, V. Antibiofilm activity of quercetin-encapsulated cytocompatible nanofibers against Candida albicans. J. Bioact. Compat. Polym. 2013, 28, 652–665. [Google Scholar] [CrossRef]
- Wei, Y.; Deng, Y.; Ma, S.; Ran, M.; Jia, Y.; Meng, J.; Han, F.; Gou, J.; Yin, T.; He, H.; et al. Local drug delivery systems as therapeutic strategies against periodontitis: A systematic review. J. Control. Release 2021, 333, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Lambris, J.D.; Hajishengallis, G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J. Oral Microbiol. 2017, 9, 1340085. [Google Scholar] [CrossRef] [Green Version]
- Toncheva, A.; Paneva, D.; Manolova, N.; Rashkov, I. Electrospun poly(L-lactide) membranes containing a single drug or multiple drug system for antimicrobial wound dressings. Macromol. Res. 2011, 19, 1310–1319. [Google Scholar] [CrossRef]
- Li, Z.; Mei, S.; Dong, Y.; She, F.; Li, Y.; Li, P.; Kong, L. Functional Nanofibrous Biomaterials of Tailored Structures for Drug Delivery-A Critical Review. Pharmaceutics 2020, 12, 522. [Google Scholar] [CrossRef]
- Wang, Y.; Murcia Valderrama, M.A.; van Putten, R.J.; Davey, C.J.E.; Tietema, A.; Parsons, J.R.; Wang, B.; Gruter, G.M. Biodegradation and Non-Enzymatic Hydrolysis of Poly(Lactic-co-Glycolic Acid) (PLGA12/88 and PLGA6/94). Polymers 2021, 14, 15. [Google Scholar] [CrossRef]
- Cavazana, T.P.; Pessan, J.P.; Hosida, T.Y.; Monteiro, D.R.; Botazzo Delbem, A.C. pH changes of mixed biofilms of Streptococcus mutans and Candida albicans after exposure to sucrose solutions in vitro. Arch. Oral Biol. 2018, 90, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Zaltsman, N.; Ionescu, A.C.; Weiss, E.I.; Brambilla, E.; Beyth, S.; Beyth, N. Surface-modified nanoparticles as anti-biofilm filler for dental polymers. PLoS ONE 2017, 12, e0189397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggenheim, B.; Giertsen, E.; Schupbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Ghasemian, A. An overview on anti-biofilm properties of quercetin against bacterial pathogens. World J. Microbiol. Biotechnol. 2019, 35, 143. [Google Scholar] [CrossRef]
- Wadia, R. Periodontitis and peri-implantitis. Br. Dent. J. 2020, 228, 422. [Google Scholar] [CrossRef] [Green Version]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharm. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Ha, A.T.; Rahmawati, L.; You, L.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-beta-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-kappaB and AP-1 Pathways. Int. J. Mol. Sci. 2021, 23, 433. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Choi, J.S.; Yi, E.H.; Lee, J.K.; Won, C.; Ye, S.K.; Kim, M.H. Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-kappaB/CRE/AP-1 signaling in murine macrophages. Mol. Cells 2013, 35, 410–420. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Florit, M.; Monjo, M.; Ramis, J.M. Quercitrin for periodontal regeneration: Effects on human gingival fibroblasts and mesenchymal stem cells. Sci. Rep. 2015, 5, 16593. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Kilmukhametova, Y.H.; Batig, V.M.; Ostafiichuk, M.A.; Tokar, O.M.; Glushchenko, T.A.; Batih, I.V.; Sheremet, M.I. Indicators of antioxidant protection of blood in necrotizing ulcerative gingivitis in experimental animals. J. Med. Life 2021, 14, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, D.E.; Hariyani, N.; Indrawati, R.; Ridwan, R.D.; Diyatri, I. Cytokines and Chemokines in Periodontitis. Eur. J. Dent. 2020, 14, 483–495. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Kumaresan, D.; Balasundaram, A.; Naik, V.K.; Appukuttan, D.P. Gingival crevicular fluid periostin levels in chronic periodontitis patients following nonsurgical periodontal treatment with low-level laser therapy. Eur. J. Dent. 2016, 10, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Kinane, D.F.; Zhang, P.; Benakanakere, M.; Singleton, J.; Biesbrock, A.; Nonnenmacher, C.; He, T. Experimental gingivitis, bacteremia and systemic biomarkers: A randomized clinical trial. J. Periodontal Res. 2015, 50, 864–869. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ouyang, X. Beyond toll-like receptors: Porphyromonas gingivalis induces IL-6, IL-8, and VCAM-1 expression through NOD-mediated NF-kappaB and ERK signaling pathways in periodontal fibroblasts. Inflammation 2014, 37, 522–533. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Luan, Q.X. Hyperresponsiveness of human gingival fibroblasts from patients with aggressive periodontitis against bacterial lipopolysaccharide. Exp. Med. 2021, 21, 417. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.T.; Liu, C.M.; Rossomando, E.F. Crevicular interleukin-1 beta in moderate and severe periodontitis patients and the effect of phase I periodontal treatment. J. Clin. Periodontol. 1995, 22, 162–167. [Google Scholar] [CrossRef]
- Yu, H.; Sun, C.; Argraves, K.M. Periodontal inflammation and alveolar bone loss induced by Aggregatibacter actinomycetemcomitans is attenuated in sphingosine kinase 1-deficient mice. J. Periodontal Res. 2016, 51, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.Y.; Hsiao, F.P.; Huang, C.Y.; Shih, C.M.; Tsao, N.W.; Tsai, C.S.; Yang, S.F.; Chang, N.C.; Hung, S.L.; Lin, Y.W. Porphyromonas gingivalis GroEL induces osteoclastogenesis of periodontal ligament cells and enhances alveolar bone resorption in rats. PLoS ONE 2014, 9, e102450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staff, P.O. Correction: 3LPS-binding protein and its interactions with P. gingivalis LPS modulate pro-inflammatory response and Toll-like receptor signaling in human oral keratinocytes. PLoS ONE 2017, 12, e0176996. [Google Scholar] [CrossRef] [Green Version]






| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| rhlA | AGCTGGGACGAATACACCA | GACTCCAGGTCGAGGAAATG |
| rhlB | GAGCGACGAACTGACCTACC | GTTGAACTTGGGGTGTACCG |
| comC | GACTTTAAAGAAATTAAGACTG | AAGCTTGTGTAAAACTTCTGT |
| comD | CTCTGATTGACCATTCTTCTGG | CATTCTGAGTTTATGCCCCTC |
| 16SrRNA | CCTACGGGAGGCAGCAGTAG | CAACAGAGCTTTACGATCCGAAA |
| 16SrRNA | CAAAACTACTGAGCTAGAGTACG | TAAGATCTCAAGGATCCCAACGGCT |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| IL-1β | TAGGGCTGGCAGAAAGGGAACA | GTGGGAGCGAATGACAGAGGGT |
| IL-6 | CGCCTTCGGTCCAGTTGCC | GCCAGTGCCTCTTTGCTGCTTT |
| IL-8 | CTCTTGGCAGCCTTCCTGATTTC | TTTTCCTTGGGGTCCAGACAGAG |
| TNF-α | AACATCCAACCTTCCCAAACGC | TGGTCTCCAGATTCCAGATGTCAGG |
| β-actin | GACTTAGTTGCGTTACACCCTTTCTTG | CTGTCACCTTCACCGTTCCAGTTTT |
| Pseudomonas aeruginosa | ||||||
| Time | PLA | PLA-QUE5 | PLA-QUE10 | |||
| pH | OD600 nm | pH | OD600 nm | pH | OD600 nm | |
| 9 h | 5.82 ± 0.33 | 1.35 ± 0.06 | 5.85 ± 0.28 | 1.37 ± 0.7 | 5.80 ± 0.38 | 1.33 ± 0.06 |
| 24 h | 5.02 ± 0.29 | 1.60 ± 0.07 | 4.95 ± 0.32 | 1.55 ± 0.06 | 5.03 ± 0.35 | 1.61 ± 0.07 |
| 48 h | 4.90 ± 0.46 | 1.65 ± 0.08 | 4.88 ± 0.43 | 1.61 ± 0.06 | 4.91 ± 0.37 | 1.66 ± 0.09 |
| 96 h | 4.83 ± 0.38 | 1.71 ± 0.06 | 4.86 ± 0.35 | 1.66 ± 0.07 | 4.81 ± 0.29 | 1.69 ± 0.07 |
| Streptococcus mutans | ||||||
| Time | PLA | PLA-QUE5 | PLA-QUE10 | |||
| pH | OD600 nm | pH | OD600 nm | pH | OD600 nm | |
| 9 h | 5.66 ± 0.45 | 0.55 ± 0.06 | 5.73 ± 0.35 | 0.35 ± 0.03 | 5.75 ± 0.41 | 0.31 ± 0.03 |
| 24 h | 4.93 ± 0.39 | 0.99 ± 0.09 | 4.98 ± 0.43 | 0.77 ± 0.03 | 4.99 ± 0.45 | 0.56 ± 0.04 |
| 48 h | 4.85 ± 0.36 | 1.35 ± 0.06 | 4.84 ± 0.29 | 1.24 ± 0.07 | 4.84 ± 0.34 | 1.03 ± 0.06 |
| 96 h | 4.79 ± 0.45 | 1.53 ± 0.07 | 4.81 ± 0.37 | 1.33 ± 0.06 | 4.82 ± 0.39 | 1.12 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Cristo, F.; Valentino, A.; De Luca, I.; Peluso, G.; Bonadies, I.; Calarco, A.; Di Salle, A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules 2022, 27, 2205. https://doi.org/10.3390/molecules27072205
Di Cristo F, Valentino A, De Luca I, Peluso G, Bonadies I, Calarco A, Di Salle A. PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment. Molecules. 2022; 27(7):2205. https://doi.org/10.3390/molecules27072205
Chicago/Turabian StyleDi Cristo, Francesca, Anna Valentino, Ilenia De Luca, Gianfranco Peluso, Irene Bonadies, Anna Calarco, and Anna Di Salle. 2022. "PLA Nanofibers for Microenvironmental-Responsive Quercetin Release in Local Periodontal Treatment" Molecules 27, no. 7: 2205. https://doi.org/10.3390/molecules27072205