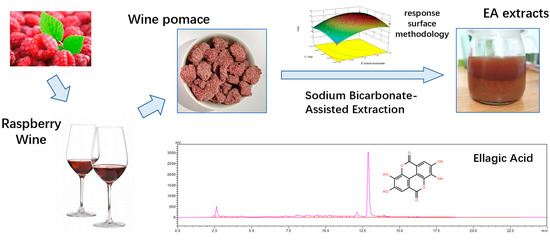
An Organic Solvent-Free Method for the Extraction of Ellagic Acid Compounds from Raspberry Wine Pomace with Assistance of Sodium Bicarbonate
Abstract
:1. Introduction
2. Results
2.1. Extraction Results with Organic Solvents
2.2. Extraction Results with Food Additive Solutions
2.3. Single Factor Assay
2.4. Response Surface Analysis
2.5. Antioxidant Activity
2.6. Quantificaiton of Ellagic Acid with HPLC Analysis
3. Discussion
3.1. The Extraction of Organic Solvents and Food Additives
3.2. Ellagic Acid Composition of the Raspberry Wine Pomace
3.3. Saponification by the Sodium Bicarbonate
3.4. Alkaline Hydrolysis Induced by the Sodium Bicarbonate Solvent
4. Materials and Methods
4.1. Sample Collection and Preparations
4.2. Reagents
4.3. Extraction with Organic Solvents
4.4. Extraction with Food Additive Solutions
4.5. Single Factor Assay
- (1)
- For the NaHCO3 concentration assay, 1 g of milled raspberry pomace was extracted by 0.1%, 0.5%, 1%, 2%, 3% NaHCO3 water solution, respectively, in an S/L ratio of 1:10 at 60 °C for 1 h shaking (200 rpm).
- (2)
- For the temperature assay, 1 g of milled raspberry pomace was extracted at 40 °C, 50 °C, 60 °C, 70 °C, 80 °C, and 90 °C, respectively, with 1% NaHCO3 solution in an S/L ratio of 1:10 for 1 h shaking (200 rpm).
- (3)
- For the time assay, 1 g of milled raspberry pomace was extracted for 5, 10, 15, 20, 25 and 30 min, respectively, with 1% NaHCO3 solution at 100 °C in an S/L ratio of 1:10 S/L ratio.
- (4)
- For the S/L ratio assay, 1 g of milled raspberry pomace was extracted in S/L ratios of 1:10, 1:50, 1:100, 1:150, and 1:200, respectively, with 1% NaHCO3 solution at 100 °C for 20 min.
4.6. Response Surface Methodology Assay
4.7. Analysis of Antioxidant Activity
4.8. HPLC Analysis of Ellagic Acid Content
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Agnieszka, M.; Michal, S.; Robert, K. Selection of Conditions of Ultrasound-Assisted, Three-Step Extraction of Ellagitannins from Selected Berry Fruit of the Rosaceae Family Using the Response Surface Methodology. Food Anal. Methods 2020, 13, 1650–1665. [Google Scholar] [CrossRef]
- Mazzoni, L.; Perez-Lopez, P.; Giampieri, F.; Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Mezzetti, B.; Battino, M. The genetic aspects of berries: From field to health. J. Sci. Food Agric. 2016, 96, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sojka, M.; Macierzynski, J.; Zaweracz, W.; Buczek, M. Transfer and Mass Balance of Ellagitannins, Anthocyanins, Flavan-3-ols, and Flavonols during the Processing of Red Raspberries (Rubus idaeus L.) to Juice. J. Agric. Food Chem. 2016, 64, 5549–5563. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Louvet, F.; Tarrade, S.; Meudec, E.; Grenier, K.; Landolt, C.; Ouk, T.S.; Bressollier, P. Enzyme-Assisted Extraction of Bioactive Compounds from Raspberry (Rubus idaeus L.) Pomace. J. Food Sci. 2019, 84, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Vrhovsek, U.; Palchetti, A.; Reniero, F.; Guillou, C.; Masuero, D.; Mattivi, F. Concentration and mean degree of polymerization of Rubus ellagitannins evaluated by optimized acid methanolysis. J. Agric. Food Chem. 2006, 54, 4469–4475. [Google Scholar] [CrossRef] [PubMed]
- Kosmala, M.; Zdunczyk, Z.; Juskiewicz, J.; Jurgonski, A.; Karlinska, E.; Macierzynski, J.; Janczak, R.; Roj, E. Chemical Composition of Defatted Strawberry and Raspberry Seeds and the Effect of These Dietary Ingredients on Polyphenol Metabolites, Intestinal Function, and Selected Serum Parameters in Rats. J. Agric. Food Chem. 2015, 63, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of Anthocyanins and Ellagitannins Following Consumption of Raspberries by Healthy Humans and Subjects with an Ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef]
- Milala, J.; Kosmala, M.; Karlinska, E.; Juskiewicz, J.; Zdunczyk, Z.; Fotschki, B. Ellagitannins from Strawberries with Different Degrees of Polymerization Showed Different Metabolism through Gastrointestinal Tract of Rats. J. Agric. Food Chem. 2017, 65, 10738–10748. [Google Scholar] [CrossRef]
- Kahkonen, M.; Kylli, P.; Ollilainen, V.; Salminen, J.-P.; Heinonen, M. Antioxidant Activity of Isolated Ellagitannins from Red Raspberries and Cloudberries. J. Agric. Food Chem. 2012, 60, 1167–1174. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, Anti-Inflammatory, and Postulated Cytotoxic Activity of Phenolic and Anthocyanin-Rich Fractions from Polana Raspberry (Rubus idaeus L.) Fruit and Juice-In Vitro Study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Kumar, S.; Bhat, Z.F.; Naqvi, Z.; Jayawardena, R. The impact of raspberry and blueberry extract on the microbial and lipid oxidative stability of calcium and chicken protein fortified composite chocolate. J. Food Process. Preserv. 2022, 46, e16216. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; Vieira do Carmo, M.A.; Wang, D.; Mocan, A.; Rodrigues Pereira, E.P.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Pina-Contreras, N.; Martinez-Moreno, A.G.; Ramirez-Anaya, J.D.P.; Espinoza-Gallardo, A.C.; Valdes, E.H.M. Raspberry (Rubus idaeus L.), a Promising Alternative in the Treatment of Hyperglycemia and Dyslipidemias. J. Med. Food 2021, 25, 121–129. [Google Scholar] [CrossRef]
- Krzepilko, A.; Prazak, R.; Swiecilo, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus Berries for the Control of Gastric Inflammation: In Vitro and In Vivo Studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [Green Version]
- Ispiryan, A.; Viskelis, J.; Viskelis, P. Red Raspberry (Rubus idaeus L.) Seed Oil: A Review. Plants 2021, 10, 994. [Google Scholar] [CrossRef]
- Szymanowska, U.; Karas, M.; Zlotek, U.; Jakubczyk, A. Effect of Fortification with Raspberry Juice on the Antioxidant and Potentially Anti-Inflammatory Activity of Wafers Subjected to In Vitro Digestion. Foods 2021, 10, 791. [Google Scholar] [CrossRef]
- Wu, D.; Chen, S.; Ye, X.; Zheng, X.; Ahmadi, S.; Hu, W.; Yu, C.; Cheng, H.; Linhardt, R.J.; Chen, J. Enzyme-extracted raspberry pectin exhibits a high-branched structure and enhanced anti-inflammatory properties than hot acid-extracted pectin. Food Chem. 2022, 383, 132387. [Google Scholar] [CrossRef]
- Klewicka, E.; Sojka, M.; Klewicki, R.; Kolodziejczyk, K.; Lipinska, L.; Nowak, A. Ellagitannins from Raspberry (Rubus idaeus L.) Fruit as Natural Inhibitors of Geotrichum candidum. Molecules 2016, 21, 908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, E.-H.; Kwon, J.-W.; Suk, K.Y.; Woong, G.S.; Lee, S.J. Comparision of Antioxidant and Antimicrobial Activity of Unripened and Ripened Black Raspberry (Rubus occidentalis) Extracts. Korean J. Food Cook. Sci. 2021, 37, 399–407. [Google Scholar]
- Ryan, T.; Wilkinson, J.M.; Cavanagh, H.M.A. Antibacterial activity of raspberry cordial in vitro. Res. Vet. Sci. 2001, 71, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, C.; Goetz, C.; Jubinville, E.; Jean, J. The New Face of Berries: A Review of Their Antiviral Proprieties. Foods 2022, 11, 102. [Google Scholar] [CrossRef]
- Shahagadkar, P.; Shah, H.; Palani, A.; Munirathinam, G. Berry derived constituents in suppressing viral infection: Potential avenues for viral pandemic management. Clin. Nutr. Espen 2021, 46, 14–20. [Google Scholar] [CrossRef]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef]
- Sham, N.; Qin, C.; Zhu, Z.; Redington, C.G.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Kou, L.; Fang, Y. Raspberry Extract with Potential Antitumor Activity Against Cervical Cancer. Anticancer Res. 2021, 41, 3343–3348. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic Acid and Its Role in Chronic Diseases. In Anti-Inflammatory Nutraceuticals and Chronic Diseases; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 928, pp. 473–479. [Google Scholar]
- Lee, J.; Kim, S.; Namgung, H.; Jo, Y.-H.; Bao, C.; Choi, H.-K.; Auh, J.-H.; Lee, H.J. Ellagic Acid Identified through Metabolomic Analysis Is an Active Metabolite in Strawberry (‘Seolhyang’) Regulating Lipopolysaccharide-Induced Inflammation. J. Agric. Food Chem. 2014, 62, 3954–3962. [Google Scholar] [CrossRef]
- Promsong, A.; Chung, W.O.; Satthakarn, S.; Nittayananta, W. Ellagic acid modulates the expression of oral innate immune mediators: Potential role in mucosal protection. J. Oral Pathol. Med. 2015, 44, 214–221. [Google Scholar] [CrossRef]
- Stoner, G.D. Whole Food Approach to Cancer Prevention: Berries as an Example. Prog. Chem. 2013, 25, 1480–1491. [Google Scholar]
- Stoner, G.D.; Wang, L.-S. Chemoprevention of Esophageal Squamous Cell Carcinoma with Berries. In Natural Products in Cancer Prevention and Therapy; Pezzuto, J.M., Suh, N., Eds.; Topics in Current Chemistry; Springer: New York, NY, USA, 2013; Volume 329, pp. 1–20. [Google Scholar]
- Aiyer, H.S.; Gupta, R.C. Berries and Ellagic Acid Prevent Estrogen-Induced Mammary Tumorigenesis by Modulating Enzymes of Estrogen Metabolism. Cancer Prev. Res. 2010, 3, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajdzanoska, M.; Petreska, J.; Stefova, M. Comparison of Different Extraction Solvent Mixtures for Characterization of Phenolic Compounds in Strawberries. J. Agric. Food Chem. 2011, 59, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Veklari, S.A.; Gordon, M.H.; Garcia-Macias, P.; Labrinea, H. Extraction and determination of ellagic acid content in chestnut bark and fruit. Food Chem. 2008, 110, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Aybastier, O.; Isik, E.; Sahin, S.; Demir, C. Optimization of ultrasonic-assisted extraction of antioxidant compounds from blackberry leaves using response surface methodology. Ind. Crops Prod. 2013, 44, 558–565. [Google Scholar] [CrossRef]
- Ayoub, M.; De Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, G.; Andlauer, W. Innovative microwave-assisted hydrolysis of ellagitannins and quantification as ellagic acid equivalents. Food Chem. 2013, 138, 2430–2434. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, J.; Luan, G.; Zhang, S.; Zhuoma, Y.; Xie, J.; Zhou, W. Quantitative Analyses of Nine Phenolic Compounds and Their Antioxidant Activities from Thirty-Seven Varieties of Raspberry Grown in the Qinghai-Tibetan Plateau Region. Molecules 2019, 24, 3932. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, G.-I.; Pilar Almajano, M. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Sojka, M.; Janowski, M.; Grzelak-Blaszczyk, K. Stability and transformations of raspberry (Rubus idaeus L.) ellagitannins in aqueous solutions. Eur. Food Res. Technol. 2019, 245, 1113–1122. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Saravanakumar, V.R.; Kulkarni, V.V.; Sivakumar, K.; Singh, A.P.; Visha, P. Yield and Quality Characteristics of Rendered Chicken Oil for Biodiesel Production. J. Am. Oil Chem. Soc. 2014, 91, 133–141. [Google Scholar] [CrossRef]
- Landbo, A.K.; Meyer, A.S. Enzyme-assisted extraction of antioxidative phenols from black current juice press residues (Ribes nigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. Food Sci. Technol. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Oxidants and Antioxidants, Part A; Packer, L., Ed.; Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 15–27. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 2 February 2022).





| No. | X1 (S/L Ratio) | X2 (NaHCO3) | X3 (Time) | Yield (mg/g) |
|---|---|---|---|---|
| 1 | 1 (1:150) | 1 (2%) | 0 (20 min) | 2.513 |
| 2 | 0 (1:100) | −1 (0%) | 1 (30 min) | 0.634 |
| 3 | 0 (1:100) | 0 (1%) | 0 (20 min) | 6.155 |
| 4 | 0 (1:100) | 0 (1%) | 0 (20 min) | 6.181 |
| 5 | 0 (1:100) | 0 (1%) | 0 (20 min) | 6.207 |
| 6 | 1 (1:150) | −1 (0%) | 0 (20 min) | 0.721 |
| 7 | 1 (1:150) | 0 (1%) | −1 (10 min) | 2.032 |
| 8 | −1 (1:150) | −1 (0%) | 0 (20 min) | 0.318 |
| 9 | 0 (1:100) | 0 (1%) | 0 (20 min) | 6.187 |
| 10 | −1 (1: 50) | 0 (1%) | 1 (30 min) | 3.014 |
| 11 | 0 (1:100) | 1 (2%) | 1 (30 min) | 5.659 |
| 12 | 1 (1:150) | 0 (1%) | 1 (30 min) | 4.061 |
| 13 | 0 (1:100) | −1 (0%) | −1 (10 min) | 0.634 |
| 14 | −1 (1: 50) | 0 (1%) | −1 (10 min) | 0.454 |
| 15 | −1 (1: 50) | 1 (2%) | 0 (20 min) | 3.479 |
| 16 | 0 (1:100) | 0 (1%) | 0 (20 min) | 5.760 |
| 17 | 0 (1:100) | 1 (2%) | −1 (10 min) | 5.219 |
| Extraction Method 1 | Ellagic Acid Yield (mg/g) | Antioxidant Activity 2 | |
|---|---|---|---|
| DPPH (μmol Trolox eq/g) | FRAP (mmol Fe(II)S eq/g) | ||
| MAW | 2.66 ± 0.35 b | 59.7 ± 3.02 b | 1.162 ± 0.080 a |
| AAW | 1.98 ± 0.12 c | 24.1 ± 0.06 c | 0.679 ± 0.032 b |
| SBAE | 6.30 ± 0.92 a | 79.0 ± 0.96 a | 0.631 ± 0.075 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, N.; Zhang, S.; Sun, S.; Wu, M.; Yang, X.; Xu, J.; Ma, K.; Guan, S.; Xu, W. An Organic Solvent-Free Method for the Extraction of Ellagic Acid Compounds from Raspberry Wine Pomace with Assistance of Sodium Bicarbonate. Molecules 2022, 27, 2145. https://doi.org/10.3390/molecules27072145
Jin N, Zhang S, Sun S, Wu M, Yang X, Xu J, Ma K, Guan S, Xu W. An Organic Solvent-Free Method for the Extraction of Ellagic Acid Compounds from Raspberry Wine Pomace with Assistance of Sodium Bicarbonate. Molecules. 2022; 27(7):2145. https://doi.org/10.3390/molecules27072145
Chicago/Turabian StyleJin, Ning, Shouyu Zhang, Shibo Sun, Minghuo Wu, Xiaojing Yang, Jianqiang Xu, Kun Ma, Shui Guan, and Weiping Xu. 2022. "An Organic Solvent-Free Method for the Extraction of Ellagic Acid Compounds from Raspberry Wine Pomace with Assistance of Sodium Bicarbonate" Molecules 27, no. 7: 2145. https://doi.org/10.3390/molecules27072145