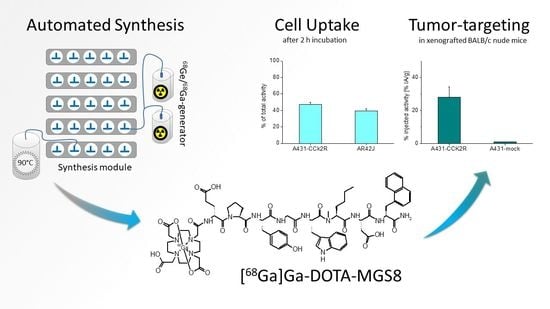
Automated Synthesis of 68Ga-Labeled DOTA-MGS8 and Preclinical Characterization of Cholecystokinin-2 Receptor Targeting
Abstract
:1. Introduction
2. Results
2.1. Radiolabeling and Quality Control
2.2. Cell Internalization Studies
2.3. Biodistribution in BALB/c Nude Mice Bearing A431-CCK2R/A431-Mock Xenografts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Radiolabeling
4.3. Analytics
4.4. Cell Internalization Study
4.5. Biodistribution in BALB/c Nude Mice Bearing A431-CCK2R/A431-Mock Xenografts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dorsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Laderach, U.; Stettler, C.; Friess, H.; Halter, F.; Schmassmann, A. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology 1997, 112, 1197–1205. [Google Scholar] [CrossRef]
- Saillan-Barreau, C.; Dufresne, M.; Clerc, P.; Sanchez, D.; Corominola, H.; Moriscot, C.; Guy-Crotte, O.; Escrieut, C.; Vaysse, N.; Gomis, R.; et al. Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes 1999, 48, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Noble, F.; Roques, B.P. CCK-B receptor: Chemistry, molecular biology, biochemistry and pharmacology. Prog. Neurobiol. 1999, 58, 349–379. [Google Scholar] [CrossRef]
- Reubi, J.C. Targeting CCK receptors in human cancers. Curr. Top. Med. Chem. 2007, 7, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Schaer, J.C.; Waser, B. Cholecystokinin(CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997, 57, 1377–1386. [Google Scholar]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Behr, T.M.; Jenner, N.; Radetzky, S.; Behe, M.; Gratz, S.; Yucekent, S.; Raue, F.; Becker, W. Targeting of cholecystokinin-B/gastrin receptors in vivo: Preclinical and initial clinical evaluation of the diagnostic and therapeutic potential of radiolabelled gastrin. Eur. J. Nucl. Med. 1998, 25, 424–430. [Google Scholar] [CrossRef]
- Klingler, M.; Hörmann, A.A.; von Guggenberg, E. Cholecystokinin-2 receptor targeting with radiolabeled peptides: Current status and future directions. Curr. Med. Chem. 2020, 27, 7112–7132. [Google Scholar] [CrossRef]
- Fani, M.; Maecke, H.R.; Okarvi, S.M. Radiolabeled peptides: Valuable tools for the detection and treatment of cancer. Theranostics 2012, 2, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Breeman, W.A.; Fröberg, A.C.; de Blois, E.; van Gameren, A.; Melis, M.; de Jong, M.; Maina, T.; Nock, B.A.; Erion, J.L.; Mäcke, H.R.; et al. Optimised labeling, preclinical and initial clinical aspects of CCK-2 receptor-targeting with 3 radiolabeled peptides. Nucl. Med. Biol. 2008, 35, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.M.; Geistlich, S.; Behe, M.; Rottenburger, C.; et al. Targeting of the Cholecystokinin-2 Receptor with the Minigastrin Analog (177)Lu-DOTA-PP-F11N: Does the Use of Protease Inhibitors Further Improve In Vivo Distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grob, N.M.; Schmid, S.; Schibli, R.; Behe, M.; Mindt, T.L. Design of Radiolabeled Analogs of Minigastrin by Multiple Amide-to-Triazole Substitutions. J. Med. Chem. 2020, 63, 4496–4505. [Google Scholar] [CrossRef]
- Maina, T.; Konijnenberg, M.W.; KolencPeitl, P.; Garnuszek, P.; Nock, B.A.; Kaloudi, A.; Kroselj, M.; Zaletel, K.; Maecke, H.; Mansi, R.; et al. Preclinical pharmacokinetics, biodistribution, radiation dosimetry and toxicity studies required for regulatory approval of a phase I clinical trial with 111In-CP04 in medullary thyroid carcinoma patients. Eur. J. Pharm Sci. 2016, 91, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Decristoforo, C.; Virgolini, I.; von Guggenberg, E. DOTA-MGS5, a New Cholecystokinin-2 Receptor-Targeting Peptide Analog with an Optimized Targeting Profile for Theranostic Use. J. Nucl. Med. 2019, 60, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Klingler, M.; Hörmann, A.A.; Rangger, C.; Desrues, L.; Castel, H.; Gandolfo, P.; von Guggenberg, E. Stabilization Strategies for Linear Minigastrin Analogues: Further Improvements via the Inclusion of Proline into the Peptide Sequence. J. Med. Chem. 2020, 63, 14668–14679. [Google Scholar] [CrossRef]
- Scemama, J.; Fourmy, D.; Zahidi, A.; Pradayrol, L.; Susini, C.; Ribet, A. Characterisation of gastrin receptors on a rat pancreatic acinar cell line (AR42J). A possible model for studying gastrin mediated cell growth and proliferation. Gut 1987, 28, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Aloj, L.; Caracò, C.; Panico, M.; Zannetti, A.; Del Vecchio, S.; Tesauro, D.; De Luca, S.; Arra, C.; Pedone, C.; Morelli, G.; et al. In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-B receptor imaging. J. Nucl. Med. 2004, 45, 485–494. [Google Scholar]
- Corlett, A.; Sani, M.A.; Van Zuylekom, J.; Ang, C.S.; von Guggenberg, E.; Cullinane, C.; Blyth, B.; Hicks, R.J.; Roselt, P.D.; Thompson, P.E.; et al. A New Turn in Peptide-Based Imaging Agents: Foldamers Afford Improved Theranostics Targeting Cholecystokinin-2 Receptor-Positive Cancer. J. Med. Chem. 2021, 64, 4841–4856. [Google Scholar] [CrossRef]
- Grob, N.M.; Haussinger, D.; Deupi, X.; Schibli, R.; Behe, M.; Mindt, T.L. Triazolo-Peptidomimetics: Novel Radiolabeled Minigastrin Analogs for Improved Tumor Targeting. J. Med. Chem. 2020, 63, 4484–4495. [Google Scholar] [CrossRef]
- Roosenburg, S.; Laverman, P.; van Delft, F.L.; Boerman, O.C. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino. Acids 2011, 41, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Peitl, P.K.; Maina, T.; Mäcke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K.; et al. Comparative biodistribution of 12 111In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloj, L.; Aurilio, M.; Rinaldi, V.; D’Ambrosio, L.; Tesauro, D.; Peitl, P.K.; Maina, T.; Mansi, R.; Von Guggenberg, E.; Joosten, L.; et al. Comparison of the binding and internalization properties of 12 DOTA-coupled and 111In-labelled CCK2/gastrin receptor binding peptides: A collaborative project under COST Action BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1417–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocak, M.; Helbok, A.; Rangger, C.; Peitl, P.K.; Nock, B.A.; Morelli, G.; Eek, A.; Sosabowski, J.K.; Breeman, W.A.; Reubi, J.C.; et al. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1426–1435. [Google Scholar] [CrossRef] [Green Version]
- Fröberg, A.C.; de Jong, M.; Nock, B.A.; Breeman, W.A.; Erion, J.L.; Maina, T.; Verdijsseldonck, M.; de Herder, W.W.; van der Lugt, A.; Kooij, P.P.; et al. Comparison of three radiolabelled peptide analogues for CCK-2 receptor scintigraphy in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Behe, M.; Behr, T.M. Cholecystokinin-13 (CCK-B)/gastrin receptor targeting peptides for staging and therapy of medullary thyroid cancer and other CCK-B receptor expressing malignancies. Biopolymers 2002, 66, 399–418. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rangger, C.; Mair, C.; Decristoforo, C.; Uprimny, C.; Virgolini, I.J.; von Guggenberg, E. Radiopharmaceutical Formulation and Preclinical Testing of Ga-68-Labeled DOTA-MGS5 for the Regulatory Approval of a First Exploratory Clinical Trial. Pharmaceuticals 2021, 14, 575. [Google Scholar] [CrossRef]
- Uprimny, C.; von Guggenberg, E.; Svirydenka, A.; Mikolajczak, R.; Hubalewska-Dydejczyk, A.; Virgolini, I.J. Comparison of PET/CT imaging with [F-18]FDOPA and cholecystokinin-2 receptor targeting [Ga-68]Ga-DOTA-MGS5 in a patient with advanced medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 935–936. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rezaeianpour, M.; Hörmann, N.; Gust, R.; Shahhosseini, S.; von Guggenberg, E. Initial In Vitro and In Vivo Evaluation of a Novel CCK2R Targeting Peptide Analog Labeled with Lutetium-177. Molecules 2020, 25, 4585. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. Improving the In Vivo Profile of Minigastrin Radiotracers: A Comparative Study Involving the Neutral Endopeptidase Inhibitor Phosphoramidon. Cancer Biother Radiopharm 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Wayua, C.; Low, P.S. Evaluation of a Nonpeptidic Ligand for Imaging of Cholecystokinin 2 Receptor-Expressing Cancers. J. Nucl. Med. 2015, 56, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Kaloudi, A.; Kanellopoulos, P.; Radolf, T.; Chepurny, O.G.; Rouchota, M.; Loudos, G.; Andreae, F.; Holz, G.G.; Nock, B.A.; Maina, T. [Tc-99m]Tc-DGA1, a Promising CCK2R-Antagonist-Based Tracer for Tumor Diagnosis with Single-Photon Emission Computed Tomography. Mol. Pharm. 2020, 17, 3116–3128. [Google Scholar] [CrossRef] [PubMed]
- Grzmil, M.; Qin, Y.; Schleuniger, C.; Frank, S.; Imobersteg, S.; Blanc, A.; Spillmann, M.; Berger, P.; Schibli, R.; Behe, M. Pharmacological inhibition of mTORC1 increases CCKBR-specific tumor uptake of radiolabeled minigastrin analogue [Lu-177]Lu-PP-F11N. Theranostics 2020, 10, 10861–10873. [Google Scholar] [CrossRef]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA Approves New (68)Ga Kit for Prostate Cancer PET. J. Nucl. Med. 2022, 63, 26N.
- Antunes, I.F.; Franssen, G.M.; Zijlma, R.; van der Woude, G.L.K.; Yim, C.B.; Laverman, P.; Boersma, H.H.; Elsinga, P.H. New Sensitive Method For HEPES Quantification in [Ga-68]-Radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, S407–S408. [Google Scholar]
- Kvaternik, H.; Plhak, E.; Rumpf, B.; Hausberger, D.; Aigner, R.M. Assay of bacterial endotoxins in radiopharmaceuticals by microplate reader. EJNMMI Radiopharm Chem. 2018, 3 (Suppl. 1), PP11. [Google Scholar]






| Quality Control | Method | Criteria | Results (n = 5) |
|---|---|---|---|
| Appearance | Visual inspection | Clear, colorless solution, free of visible particles | Conforms |
| pH value | pH indicator strip | 4–8 | 7 |
| Volume | Graduated vial | 15–20 mL | 16–17 mL |
| Radioactivity concentration | Dose calibrator | ≥22.2 MBq/mL | 37.9 ± 8.7 MBq/mL |
| Radionuclide identity | Gamma-ray spectrometry | 511 keV; 1022 keV | Conforms |
| Radionuclide identity | Half-life | 62–74 min | 68.2 ± 1.0 min |
| Identity of [68Ga]Ga-DOTA-MGS8 (comparison with reference) | HPLC | 14.4–16.0 min | 14.9 ± 0.1 min |
| Percentage of free gallium-68 (RRT 0.1–0.3) | Radio-HPLC | ≤2% | 0.5 ± 0.2% |
| Radiochemical impurities with RRT 0.45–0.95 and 1.05–1.20 | Radio-HPLC | ≤8% | 6.7 ± 0.5% |
| Percentage of [68Ga]Ga-DOTA-MGS8 | Radio-HPLC (T) | >92% | 92.8 ± 0.6% |
| Radionuclide incorporation (retardation factor >0.8) | Radio-iTLC (A) | ≥97% | 99.4 ± 0.2% |
| Radiochemical purity | RCP = A × (T/100) | ≥91% | 92.2 ± 0.8% |
| DOTA-MGS8, [68Ga]Ga-DOTA-MGS8 and related substances (RRT 0.6–1.4) | HPLC | ≤50 µg/V | 38.9 ± 4.7 µg/V |
| Quality Control | Method | Criteria | Results (n = 3) |
|---|---|---|---|
| Ethanol content | Gas chromatography | ≤10% (v/v) | Conforms |
| HEPES content | HPLC | ≤500 µg/V | ≤20 |
| Radionuclide purity | Gamma-ray spectrometry | Ge-68 ≤ 0.001% (after >48 h) | Conforms |
| Bacterial endotoxins | LAL test | ≤175 IU/V | ≤40 |
| Sterility | Ph. Eur. | Sterile | Conforms |
| Tumor-to-Normal Tissue Ratio | |
|---|---|
| Tumor-to-blood | 13.38 ± 1.77 |
| Tumor-to-kidney | 4.41 ± 0.40 |
| Tumor-to-stomach | 11.03 ± 2.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörmann, A.A.; Plhak, E.; Klingler, M.; Rangger, C.; Pfister, J.; Schwach, G.; Kvaternik, H.; von Guggenberg, E. Automated Synthesis of 68Ga-Labeled DOTA-MGS8 and Preclinical Characterization of Cholecystokinin-2 Receptor Targeting. Molecules 2022, 27, 2034. https://doi.org/10.3390/molecules27062034
Hörmann AA, Plhak E, Klingler M, Rangger C, Pfister J, Schwach G, Kvaternik H, von Guggenberg E. Automated Synthesis of 68Ga-Labeled DOTA-MGS8 and Preclinical Characterization of Cholecystokinin-2 Receptor Targeting. Molecules. 2022; 27(6):2034. https://doi.org/10.3390/molecules27062034
Chicago/Turabian StyleHörmann, Anton Amadeus, Elisabeth Plhak, Maximilian Klingler, Christine Rangger, Joachim Pfister, Gert Schwach, Herbert Kvaternik, and Elisabeth von Guggenberg. 2022. "Automated Synthesis of 68Ga-Labeled DOTA-MGS8 and Preclinical Characterization of Cholecystokinin-2 Receptor Targeting" Molecules 27, no. 6: 2034. https://doi.org/10.3390/molecules27062034