A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications
Abstract
:1. Introduction
2. Results
2.1. Literature Search Results
2.2. Hypoglycemic Activity
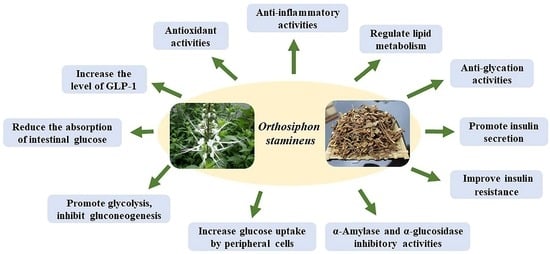
2.3. Mechanisms of O. stamineus in the Treatment of Diabetes
2.3.1. Antioxidant Activity
2.3.2. Anti-Inflammatory Activity
2.3.3. Regulate Lipid Metabolism
2.3.4. Inhibit the Activities of α-Amylase and α-Glucosidase
2.3.5. Promote Insulin Secretion, Ameliorate Insulin Resistance, Enhance Insulin Sensitivity
2.3.6. Reduce the Absorption of Intestinal Glucose, Increase Glucose Uptake by Peripheral Cells
2.3.7. Promote Glycolysis, Inhibit Gluconeogenesis
2.3.8. Increase the Level of GLP-1
2.4. Mechanisms of O. stamineus in the Treatment of Diabetic Complications
2.5. Toxicity
3. Clinical Applications
4. The Pharmacodynamic Material Basis
4.1. Phenolic Acids
4.2. Flavonoids
4.3. Triterpenoids
5. Discussion
6. Methods
6.1. Search Strategy
6.2. Eligibility Criteria
6.3. Data Extraction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shafaei, A.; Halim, N.H.A.; Zakaria, N.; Ismail, Z. Analysis of Free Amino Acids in Different Extracts of Orthosiphon stamineus Leaves by High-Performance Liquid Chromatography Combined with Solid-Phase Extraction. Pharmacogn. Mag. 2017, 13 (Suppl. S3), 385–391. [Google Scholar]
- Guan, S.C.; Fan, G.Y. Diterpenoids from Aerial Parts of Clerodendranthus spicatus and Their Cytotoxic Activity. Helv. Chim. Acta 2014, 97, 1708–1713. [Google Scholar] [CrossRef]
- Zhou, H.C.; Yang, L.; Guo, R.Z.; Li, J. Phenolic acid derivatives with neuroprotective effect from the aqueous extract of Clerodendranthus spicatus. J. Asian Nat. Prod. Res. 2017, 19, 974–980. [Google Scholar] [CrossRef]
- Gan, S.H.; Tham, T.C.; Ng, M.X.; Chua, L.S.; Aziz, R.; Baba, M.R.; Abdullah, L.C.; Ong, S.P.; Law, C.L. Study on retention of metabolites composition in misai kucing (Orthosiphon stamineus) by heat pump assisted solar drying. J. Food Processing Preserv. 2017, 41, e13262. [Google Scholar] [CrossRef]
- Luo, Y.; Cheng, L.Z.; Luo, Q.; Yan, Y.M.; Wang, S.M.; Sun, Q.; Cheng, Y.X. New ursane-type triterpenoids from Clerodendranthus spicatus. Fitoterapia 2017, 119, 69–74. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, Q.; Ma, G.; Zhang, X.; Yuan, J.; Wu, H.; Liu, H.; Yang, J.; Xu, X. Four new phenolic acids from Clerodendranthus spicatus. Phytochem. Lett. 2014, 8, 16–21. [Google Scholar] [CrossRef]
- Robaina-Mesa, M.; López-Hernández, O.D.; Rodríguez-Chanfrau, J.E.; Nogueira-Mendoza, A. Spray dried aqueous extract of Orthosiphon aristatus Blume (Java tea). Braz. J. Pharm. Sci. 2017, 53, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.S.; Choo, B.K.M.; Ahmed, P.K.; Othman, I.; Shaikh, M.F. A Systematic Review of the Protective Actions of Cat’s Whiskers (Misai Kucing) on the Central Nervous System. Front. Pharm. 2020, 11, 692. [Google Scholar] [CrossRef]
- Yuliana, N.D.; Khatib, A.; Link-Struensee, A.M.R.; Ijzerman, A.P.; Rungkat-Zakaria, F.; Choi, Y.H.; Verpoorte, R. Adenosine A(1) Receptor Binding Activity of Methoxy Flavonoids from Orthosiphon stamineus. Planta Med. 2009, 75, 132–136. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sugawara, K.; Saito, M.; Saito, S.; Murakami, S.; Miyata, N.; Kawashima, A.; Morimoto, S.; Gao, N.; Zhang, X.G.; et al. In vitro TGF-beta 1 antagonistic activity of ursolic and oleanolic acids isolated from Clerodendranthus spicatus. Planta Med. 2003, 69, 673–675. [Google Scholar]
- Hashim, S.; Beh, H.K.; Hamil, M.S.R.; Ismail, Z.; Majid, A. High-performance thin-layer chromatography method development, validation, and simultaneous quantification of four compounds identified in standardized extracts of Orthosiphon stamineus. Pharmacogn. Res. 2016, 8, 238–243. [Google Scholar]
- Saidan, N.H.; Aisha, A.F.; Hamil, M.S.; Majid, A.M.; Ismail, Z. A novel reverse phase high-performance liquid chromatography method for standardization of Orthosiphon stamineus leaf extracts. Pharmacogn. Res. 2015, 7, 23–31. [Google Scholar]
- Heryanto, R.; Pradono, D.I.; Marlina, E.; Darusman, L.K. Classification of java tea (Orthosiphon aristatus) quality using FTIR spectroscopy and chemometrics. In International Symposium on Bioinformatics, Chemometrics and Metabolomics; Kusuma, W.A., Rohman, A., Putri, S.P., Eds.; Iop Publishing Ltd.: Bristol, UK, 2017; Volume 835. [Google Scholar]
- Febriani, Y.; Fidrianny, I.; Elfahmi. Isolation of two methoxy flavonoid compounds from kumis kucing (Orthoshipon stamineus, Benth.) a popular plant in Indonesian herbal medicine Jamu. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 1640–1646. [Google Scholar]
- Li, Y.M.; Xiang, B.; Li, X.Z.; Yan, Y.M.; Cheng, Y.X. New Diterpenoids from Clerodendranthus spicatus. Nat. Prod. Bioprospect. 2017, 7, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.X.; Zhang, X.P.; Li, P.F.; Sun, Z.H.; Zhu, N.L.; Zhu, Y.D.; Yang, J.S.; Chen, D.L.; Wu, H.F.; Xu, X.D. Four new phenolic acid with unusual bicycle [2.2.2] octane moiety from Clerodendranthus spicatus and their anti-inflammatory activity. Fitoterapia 2015, 105, 61–65. [Google Scholar] [CrossRef]
- American Diabetes Association, Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. S1), 81–90. [CrossRef] [Green Version]
- Federation, I.D. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Prabhakar, P.K.; Doble, M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin. J. Integr Med. 2011, 17, 563–574. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An update on natural compounds in the remedy of diabetes mellitus: A systematic review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharm. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Wehmeier, U.F.; Piepersberg, W. Biotechnology and molecular biology of the alpha-glucosidase inhibitor acarbose. Appl. Microbiol. Biotechnol. 2004, 63, 613–625. [Google Scholar] [CrossRef]
- Furman, B.L. The development of Byetta (exenatide) from the venom of the Gila monster as an anti-diabetic agent. Toxicon 2012, 59, 464–471. [Google Scholar] [CrossRef]
- Fu, Y. Historical story on natural medicinal chemistry: 60 years history of metformin, a classical antidiabetic drug originated from Galega officinalis. Chin. Tradit. Herbal Drugs 2017, 48, 4591–4600. [Google Scholar]
- Wang, S.; Lu, A.; Zhang, L.; Shen, M.; Xu, T.; Zhan, W.; Jin, H.; Zhang, Y.; Wang, W. Extraction and purification of pumpkin polysaccharides and their hypoglycemic effect. Int. J. Biol. Macromol. 2017, 98, 182–187. [Google Scholar] [CrossRef]
- Zhao, R.; Jin, R.; Chen, Y.; Han, F.-M. Hypoglycemic and Hypolipidemic Effects of Lycium barbarum Polysaccharide in Diabetic Rats. Chin. Herb. Med. 2015, 7, 310–315. [Google Scholar] [CrossRef]
- Bai, Y.; Zang, X.; Ma, J.; Xu, G. Anti-Diabetic Effect of Portulaca oleracea L. Polysaccharideandits Mechanism in Diabetic Rats. Int. J. Mol. Sci. 2016, 17, 1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Sun, Z.; Zhang, X.; Yuan, J.; Wu, H.; Yang, J.; Xu, X. Clerodendranoic acid, a new phenolic acid from Clerodendranthus spicatus. Molecules 2012, 17, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Mizanur Rahman, S.M. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab. J. Chem. 2015, 8, 218–221. [Google Scholar] [CrossRef]
- Nguyen, M.T.T.; Awale, S.; Tezuka, Y.; Chien-Hsiung, C.; Kadota, S. Staminane- and isopimarane-type diterpenes from Orthosiphon stamineus of Taiwan and their nitric oxide inhibitory activity. J. Nat. Prod. 2004, 67, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Adnyana, I.K.; Kadota, S. Highly-oxygenated isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia and their nitric oxide inhibitory activity. Chem. Pharm. Bull. 2003, 51, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Ismail, Z. Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus. Arab. J. Chem. 2013, 6, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Adam, Y.; Somchit, M.N.; Sulaiman, M.R.; Nasaruddin, A.A.; Zuraini, A.; Bustamam, A.A.; Zakaria, Z.A. Diuretic properties of Orthosiphon stamineus Benth. J. Ethnopharmacol. 2009, 124, 154–158. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Yam, M.F.; Ang, L.F.; Mohamed, A.J.; Asmawi, M.Z. Antidiabetic Properties and Mechanism of Action of Orthosiphon stamineus Benth Bioactive Sub-fraction in Streptozotocin-induced Diabetic Rats. J. Acupunct. Meridian Stud. 2013, 6, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameer, O.Z.; Salman, I.M.; Asmawi, M.Z.; Ibraheem, Z.O.; Yam, M.F. Orthosiphon stamineus: Traditional uses, phytochemistry, pharmacology, and toxicology. J. Med. Food 2012, 15, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, K.; Sultan, S.; Adam, A. Orthosiphon stamineus Benth. is an Outstanding Food Medicine: Review of Phytochemical and Pharmacological Activities. J. Pharm. Bioallied. Sci. 2018, 10, 109–118. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Y.; Wen, Q.; Feng, Y.; Tan, T. Comprehensive chemical and metabolic profiling of anti-hyperglycemic active fraction from Clerodendranthi Spicati Herba. J. Sep. Sci. 2021, 44, 1824–1832. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Mohamed, Z. Antiobesity and Lipid Lowering Effects of Orthosiphon stamineus in High-Fat Diet-Induced Obese Mice. Planta Med. 2017, 83, 684–692. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Ahmad, M.; Ang, L.F.; Asmawi, M.Z.; Yam, M.F. Evaluation of alpha-Glucosidase Inhibitory Effect of 50% Ethanolic Standardized Extract of Orthosiphon stamineus Benth in Normal and Streptozotocin-Induced Diabetic Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 754931. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, E.A.H.; Mohamed, A.J.; Asmawi, M.Z.; Sadikun, A.; Ebrika, O.S.; Yam, M.F. Antihyperglycemic effect of orthosiphon stamineus benth leaves extract and its bioassay-guided fractions. Molecules 2011, 16, 3787–3801. [Google Scholar] [CrossRef] [Green Version]
- Sriplang, K.; Adisakwattana, S.; Rungsipipat, A.; Yibchok-anun, S. Effects of Orthosiphon stamineus aqueous extract on plasma glucose concentration and lipid profile in normal and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007, 109, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Screening of antidiabetic and antioxidant activities of medicinal plants. J. Integr. Med. 2015, 13, 297–305. [Google Scholar] [CrossRef]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and Antidiabetic Effects of Natural Polyphenols and Isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batubara, I.; Komariah, K.; Sandrawati, A.; Nurcholis, W. Genotype selection for phytochemical content and pharmacological activities in ethanol extracts of fifteen types of Orthosiphon aristatus (Blume) Miq. leaves using chemometric analysis. Sci. Rep. 2020, 10, 20945. [Google Scholar] [CrossRef]
- Lau, C.H.; Chua, L.S. Solvation Free Energy Simulation for Rosmarinic Acid Extraction from Orthosiphon stamineus. Methods Protoc. 2019, 2, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, F.L.; Yam, M.F.; Asmawi, M.Z.; Chan, L.-K. Elicitation of Orthosiphon stamineus cell suspension culture for enhancement of phenolic compounds biosynthesis and antioxidant activity. Ind. Crops Prod. 2013, 50, 436–442. [Google Scholar] [CrossRef]
- Chua, L.S.; Lau, C.H.; Chew, C.Y.; Ismail, N.I.M.; Soontorngun, N. Phytochemical profile of Orthosiphon aristatus extracts after storage: Rosmarinic acid and other caffeic acid derivatives. Phytomedicine 2018, 39, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.F.; Hashim, Z.; Soon, W.T.; Rahman, N.S.A.; Zainudin, A.N.; Majid, F.A.A. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Tradit. Complement. Med. 2017, 7, 452–465. [Google Scholar] [CrossRef]
- Deetae, P.; Parichanon, P.; Trakunleewatthana, P.; Chanseetis, C.; Lertsiri, S. Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chem. 2012, 133, 953–959. [Google Scholar] [CrossRef]
- Akowuah, G.A.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Cai, X.; Xiao, C.; Xue, H.; Xiong, H.; Hang, Y.; Xu, J.; Lu, Y. A comparative study of the antioxidant and intestinal protective effects of extracts from different parts of Java tea (Orthosiphon stamineus). Food Sci. Nutr. 2018, 6, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [PubMed] [Green Version]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Chen, W.-D.; Zhao, Y.-L.; Dai, Z.; Zhou, Z.-S.; Zhu, P.-F.; Liu, Y.-P.; Zhao, L.-X.; Luo, X.-D. Bioassay-guided isolation of anti-inflammatory diterpenoids with highly oxygenated substituents from kidney tea (Clerodendranthus spicatus). J. Food Biochem. 2020, 44, e13511. [Google Scholar] [CrossRef]
- Awale, S.; Tezuka, Y.; Kobayashi, M.; Ueda, J.-Y.; Kadota, S. Neoorthosiphonone A; a nitric oxide (NO) inhibitory diterpene with new carbon skeleton from Orthosiphon stamineus. Tetrahedron Lett. 2004, 45, 1359–1362. [Google Scholar] [CrossRef]
- Awale, S.; Tezuka, Y.; Banskota, A.H.; Kadota, S. Siphonols A–E: Novel nitric oxide inhibitors from Orthosiphon stamineus of Indonesia. Bioorg. Med. Chem. Lett. 2003, 13, 31–35. [Google Scholar] [CrossRef]
- Jaiswal, M.; Schinske, A.; Pop-Busui, R. Lipids and lipid management in diabetes. Best Pr. Res. Clin. Endocrinol. Metab. 2014, 28, 325–338. [Google Scholar] [CrossRef]
- Mosavat, M.; Mirsanjari, M.; Arabiat, D.; Smyth, A.; Whitehead, L. The Role of Sleep Curtailment on Leptin Levels in Obesity and Diabetes Mellitus. Obes. Facts 2021, 14, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Howlader, M.; Sultana, M.I.; Akter, F.; Hossain, M.M. Adiponectin gene polymorphisms associated with diabetes mellitus: A descriptive review. Heliyon 2021, 7, e07851. [Google Scholar] [CrossRef]
- Lokman, E.F.; Saparuddin, F.; Muhammad, H.; Omar, M.H.; Zulkapli, A. Orthosiphon stamineus as a potential antidiabetic drug in maternal hyperglycemia in streptozotocin-induced diabetic rats. Integr. Med. Res. 2019, 8, 173–179. [Google Scholar] [CrossRef]
- Azam, A.A.; Pariyani, R.; Ismail, I.S.; Ismail, A.; Khatib, A.; Abas, F.; Shaari, K. Urinary metabolomics study on the protective role of Orthosiphon stamineus in Streptozotocin induced diabetes mellitus in rats via H-1 NMR spectroscopy. BMC Complement. Altern. Med. 2017, 17, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, X.; Sun, B.; Li, W.; Zhang, X.; Zhao, Y. Anti-Diabetic Activity Phenolic Constituents from Red Wine Against α-Glucosidase and α-Amylase. J. Food Processing Preserv. 2017, 41, e12942. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yen, Y.Y.; Hung, K.C.; Hsu, S.W.; Lan, S.J.; Lin, H.C. Inhibitory effects of pu-erh tea on alpha glucosidase and alpha amylase: A systemic review. Nutr. Diabetes 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Niu, H.; Nie, A.; Bian, M. Bioactivity-guided separation of potential alpha-glycosidase inhibitor from clerodendranthus spicatus based on HSCCC coupled with molecular docking. Sci. Rep. 2021, 11, 6914. [Google Scholar] [CrossRef]
- Thengyai, S.; Thiantongin, P.; Sontimuang, C.; Ovatlarnporn, C.; Puttarak, P. α-Glucosidase and α-amylase inhibitory activities of medicinal plants in Thai antidiabetic recipes and bioactive compounds from Vitex glabrata R. Br. stem bark. J. Herb. Med. 2020, 19, 100302. [Google Scholar] [CrossRef]
- Mohamed, E.A.H.; Siddiqui, M.J.A.; Ang, L.F.; Sadikun, A.; Chan, S.H.; Tan, S.C.; Asmawi, M.Z.; Yam, M.F. Potent alpha-glucosidase and alpha-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complement. Altern. Med. 2012, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Schofield, C.J.; Sutherland, C. Disordered insulin secretion in the development of insulin resistance and Type 2 diabetes. Diabet. Med. 2012, 29, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [Green Version]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, e1. [Google Scholar] [CrossRef] [Green Version]
- Bakke, J.; Haj, F.G. Protein-tyrosine phosphatase 1B substrates and metabolic regulation. Semin. Cell Dev. Biol. 2015, 37, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Xie, L.; Yang, F.; Wang, W.; Zhou, Q.; Xiang, M.; Zhou, S.; Lv, W.; Jia, Y.; Pokhrel, L.; et al. Recent advance on PTP1B inhibitors and their biomedical applications. Eur. J. Med. Chem. 2020, 199, 112376. [Google Scholar] [CrossRef] [PubMed]
- Phi Hung, N.; Huynh Nhu, T.; Duc Thuan, H.; Quoc Trung, V.; Minh Quan, P.; Manh Hung, T.; Dao Cuong, T. Glucose Uptake Stimulatory and PTP1B Inhibitory Activities of Pimarane Diterpenes from Orthosiphon stamineus Benth. Biomolecules 2019, 9, 859. [Google Scholar]
- Lee, H.-J.; Choi, Y.-J.; Park, S.-Y.; Kim, J.-Y.; Won, K.-C.; Son, J.-K.; Kim, Y.-W. Hexane Extract of Orthosiphon stamineus Induces Insulin Expression and Prevents Glucotoxicity in INS-1 Cells. Diabetes Metab. J. 2015, 39, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.I.; Goodyear, L.J. Exercise and type 2 diabetes: Molecular mechanisms regulating glucose uptake in skeletal muscle. Adv. Physiol. Educ. 2014, 38, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Khattak, M.M.A.K.; Taher, M.; Ichwan, S.J.A.; Azahari, N. Selected Herbal Extracts Improve Diabetes Associated Factors in 3T3-L1 Adipocytes. Procedia—Soc. Behav. Sci. 2013, 91, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Azahari, N.; Khattak, M.M.A.K.; Taher, M.; Ichwan, S.J.A. Herbal extracts exhibit anti-diabetic activities in 3T3-L1 adipocytes model. Prog. Nutr. 2015, 17, 301–310. [Google Scholar]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Ostergaard, L.; Frandsen, C.S.; Madsbad, S. Treatment potential of the GLP-1 receptor agonists in type 2 diabetes mellitus: A review. Expert Rev. Clin. Pharm. 2016, 9, 241–265. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic potential of curcumin in diabetic complications. Pharm. Res. 2018, 136, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Huang, R.G.; Zheng, X.Z.; Chen, J.H.; Li, Y.T. Study on the protective effect of Kidney tea on kidney of diabetic rats and its mechanism. Chin. J. Integr. Tradit. West. Nephrol. 2007, 8, 32–34. [Google Scholar]
- Pariyani, R.; Ismail, I.S.; Azam, A.A.; Abas, F.; Shaari, K.; Sulaiman, M.R. Phytochemical Screening and Acute Oral Toxicity Study of Java Tea Leaf Extracts. Biomed Res. Int. 2015, 2015, 742420. [Google Scholar] [CrossRef] [Green Version]
- Han, C.J.; Hussin, A.H.; Ismail, S. Toxicity study of Orthosiphon stamineus Benth (Misai Kucing) on Sprague Dawley rats. Trop. Biomed. 2008, 25, 9–16. [Google Scholar]
- Mohamed, E.A.H.; Lim, C.P.; Ebrika, O.S.; Asmawi, M.Z.; Sadikun, A.; Yam, M.F. Toxicity evaluation of a standardised 50% ethanol extract of Orthosiphon stamineus. J. Ethnopharmacol. 2011, 133, 358–363. [Google Scholar] [CrossRef]
- Muhammad, H.; Sulaiman, S.A.; Ismail, Z.; Paumgartten, F.J.R. Study on the developmental toxicity of a standardized extract of in rats Orthosiphon stamineus. Rev. Bras. Farmacogn. 2013, 23, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, H.; Gomes-Carneiro, M.R.; Poça, K.S.; De-Oliveira, A.C.A.X.; Afzan, A.; Sulaiman, S.A.; Ismail, Z.; Paumgartten, F.J.R. Evaluation of the genotoxicity of Orthosiphon stamineus aqueous extract. J. Ethnopharmacol. 2011, 133, 647–653. [Google Scholar] [CrossRef]
- Li, J.Y.; Kang, L.Q. Research Progress in Exploitation and Utilization of Clerodendranthus spicatus. Acta Agric. Jiangxi 2010, 22, 99–104. [Google Scholar]
- Song, L.Q.; Pei, C.P.; Song, Y.X. Clinical Study on the Treatment of Diabetic Nephropathy with Cordyceps Sinensis and Kidney Tea Decoction. Inf. Tradit. Chin. Med. 2009, 26, 38–39. [Google Scholar]
- Song, L.Q.; Jin, L.; Song, Y. The Clinical Study of the Effect of Diabetic Nephropathy Treated by Chongcao Shencha Capsule. Chin. Arch. Tradit. Chin. Med. 2009, 27, 679–681. [Google Scholar]
- Yu, S. Effects of Chongcao Shencha Capsule on Expression of the ECM and TIMP-1mRNA in Rat Glomerulosclerosis. Doctoral Thesis, Heilongjiang University of Chinese Medicine, Harbin, China, 2008. [Google Scholar]
- Xu, Y. The Experimental Study and Clinical Observation on the Effects of Chongcaoshencha Prescription Inflencing Early Changes of Chronic Renal Failure. Doctoral Thesis, Heilongjiang University of Chinese Medicine, Harbin, China, 2007. [Google Scholar]
- Vinothiya, K.; Ashokkumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed Pharm. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Song, Y.; Wu, T.; Yang, Q.; Chen, X.; Wang, M.; Wang, Y.; Peng, X.; Ou, S. Ferulic acid alleviates the symptoms of diabetes in obese rats. J. Funct. Foods 2014, 9, 141–147. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Ignacimuthu, S.; Paulraj, M.G.; Sasikumar, P. Antihyperglycemic activity and antidiabetic effect of methyl caffeate isolated from Solanum torvum Swartz. fruit in streptozotocin induced diabetic rats. Eur. J. Pharm. 2011, 670, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ma, F.; Zhao, D.; Xue, Z. Exploring the effect of salvianolic acid C on α-glucosidase: Inhibition kinetics, interaction mechanism and molecular modelling methods. Process Biochem. 2019, 78, 178–188. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, N.; Schmatz, R.; Ahmed, M.; Pereira, L.B.; da Costa, P.; Reichert, K.P.; Dalenogare, D.; Pelinson, L.P.; Vieira, J.M.; Stefanello, N.; et al. Protective effect of rosmarinic acid against oxidative stress biomarkers in liver and kidney of strepotozotocin-induced diabetic rats. J. Physiol. Biochem. 2015, 71, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Huang, J.; Zhao, D.; Du, B.; Wang, M. Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food Funct. 2018, 9, 851–860. [Google Scholar] [CrossRef]
- Wen, Y.J.; Yin, M.C. The anti-inflammatory and anti-glycative effects of rosmarinic acid in the livers of type 1 diabetic mice. Biomedicine 2017, 7, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Mahesh, R.; Sachinkumar, T.; Swati, K. Antiobesity, antihyperlipidemic and antidiabetic agents of protocatechuic acid in high fatty diet along with alloxan induced diabetes. Int. J. Pharm. Sci. Res. 2019, 10, 1742–1746. [Google Scholar]
- Harini, R.; Pugalendi, K.V. Antioxidant and antihyperlipidaemic activity of protocatechuic acid on streptozotocindiabetic rats. Redox Rep. 2013, 15, 71–80. [Google Scholar] [CrossRef]
- Runtuwene, J.; Cheng, K.C.; Asakawa, A.; Amitani, H.; Amitani, M.; Morinaga, A.; Takimoto, Y.; Kairupan, B.H.; Inui, A. Rosmarinic acid ameliorates hyperglycemia and insulin sensitivity in diabetic rats, potentially by modulating the expression of PEPCK and GLUT4. Drug Des. Devel. 2016, 10, 2193–2202. [Google Scholar]
- Unger, J.R.; Parkin, C.G. Glucagon-like peptide-1 (GLP-1) receptor agonists: Differentiating the new medications. Diabetes 2011, 2, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, S.; Karuppiah, M.; Thiruppathi, M. Antihyperglycaemic potential of rosmarinic acid attenuates glycoprotein moiety in high-fat diet and streptozotocin-induced diabetic rats. All Life 2020, 13, 120–130. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.L.; Xu, Y.; Zhang, S.P.; Hou, J.; Zhu, H.B. Effect of rosmarinic acid on experimental diabetic nephropathy. Basic Clin. Pharm. Toxicol. 2012, 110, 390–395. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Dua, T.K.; Khanra, R.; Joardar, S.; Nandy, A.; Saha, A.; de Feo, V.; Dewanjee, S. Protocatechuic Acid, a Phenolic from Sansevieria roxburghiana Leaves, Suppresses Diabetic Cardiomyopathy via Stimulating Glucose Metabolism, Ameliorating Oxidative Stress, and Inhibiting Inflammation. Front. Pharmacol. 2017, 8, 251. [Google Scholar] [CrossRef]
- Jin, C.J.; Yu, S.H.; Wang, X.M.; Woo, S.J.; Park, H.J.; Lee, H.C.; Choi, S.H.; Kim, K.M.; Kim, J.H.; Park, K.S.; et al. The effect of lithospermic acid, an antioxidant, on development of diabetic retinopathy in spontaneously obese diabetic rats. PLoS ONE 2014, 9, e98232. [Google Scholar]
- Pei, Y.; Dong, X.; Tang, X.Y.; He, A.H.; Yang, H.; Zhou, H.; Zou, J.Z.; Li, H.N. Influence of Caffeic Acid on blood glucose concentration in rats with type 2 diabetes mellitus. China Mod. Med. 2018, 25, 16–18. [Google Scholar]
- Sotnikova, R.; Okruhlicova, L.; Vlkovicova, J.; Navarova, J.; Gajdacova, B.; Pivackova, L.; Fialova, S.; Krenek, P. Rosmarinic acid administration attenuates diabetes-induced vascular dysfunction of the rat aorta. J. Pharm. Pharm. 2013, 65, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, W.; Zhang, J.; Xie, M.; Wang, X. Baicalein improves glucose metabolism in insulin resistant HepG2 cells. Eur. J. Pharmacol. 2019, 854, 187–193. [Google Scholar] [CrossRef]
- Sarkar, P.; Nath, K.; Banu, S. Modulatory effect of baicalein on gene expression and activity of antioxidant enzymes in streptozotocin-nicotinamide induced diabetic rats. Braz. J. Pharm. Sci. 2019, 55, e18201. [Google Scholar] [CrossRef]
- Annadurai, T.; Muralidharan, A.R.; Joseph, T.; Hsu, M.J.; Thomas, P.A.; Geraldine, P. Antihyperglycemic and antioxidant effects of a flavanone, naringenin, in streptozotocin-nicotinamide-induced experimental diabetic rats. J. Physiol. Biochem. 2012, 68, 307–318. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, M.Y.; Bhakta, H.K.; Min, B.S.; Choi, J.S. Prunin is a highly potent flavonoid from Prunus davidiana stems that inhibits protein tyrosine phosphatase 1B and stimulates glucose uptake in insulin-resistant HepG2 cells. Arch. Pharm. Res. 2017, 40, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; He, Y.; Ji, L.L.; Wang, K.Y.; Wang, Y.L.; Chen, E.F.; Geng, Y.; OuYang, P.; Lai, W.M. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 2017, 8, 101545–101559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Raj, V.; Keshari, A.K.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPARgamma/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018, 280, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Jayakumar, M.; Thirumurugan, K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods 2015, 14, 363–373. [Google Scholar] [CrossRef]
- Sirovina, D.; Orsolic, N.; Gregorovic, G.; Koncic, M.Z. Naringenin ameliorates pathological changes in liver and kidney of diabetic mice: A preliminary study. Arh. Hig. Rada. Toksikol. 2016, 67, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Ahmed, F.; Banerjee, S.; Saha, U. Naringenin ameliorates streptozotocin-induced diabetic rat renal impairment by downregulation of TGF-β1 and IL-1 via modulation of oxidative stress correlates with decreased apoptotic events. Pharm. Biol. 2016, 54, 1616–1627. [Google Scholar] [CrossRef] [Green Version]
- Ahad, A.; Mujeeb, M.; Ahsan, H.; Siddiqui, W.A. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie 2014, 106, 101–110. [Google Scholar] [CrossRef]
- Tsai, S.J.; Huang, C.S.; Mong, M.C.; Kam, W.Y.; Huang, H.Y.; Yin, M.C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem 2012, 60, 514–521. [Google Scholar] [CrossRef]
- Singh, P.; Bansal, S.; Kuhad, A.; Kumar, A.; Chopra, K. Naringenin ameliorates diabetic neuropathic pain by modulation of oxidative-nitrosative stress, cytokines and MMP-9 levels. Food Funct. 2020, 11, 4548–4560. [Google Scholar] [CrossRef]
- Zaidun, N.H.; Sahema, Z.C.T.; Mardiana, A.A.; Santhana, R.L.; Latiff, A.A.; Syed Ahmad Fuad, S.B. Effects of naringenin on vascular changes in prolonged hyperglycaemia in fructose-STZ diabetic rat model. Drug Discov. Ther. 2019, 13, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Sun, J.; Zhang, B.; Xing, Y.; Yu, X.; Li, X.; Xiu, Z.; Dong, Y. Baicalein improves insulin resistance via regulating SOCS3 and enhances the effect of acarbose on diabetes prevention. J. Funct. Foods 2017, 37, 339–353. [Google Scholar] [CrossRef]
- Wei, X.F.; Lin, S.B.; Xiong, H.P.; Yang, Y.; Zhou, Q.; Xu, J. The Influence of Baicalein on Islet Function in Diabetic Rats. Chin. J. Integr. Med. Cardio-Cerebrovasc. Dis. 2019, 17, 2933–2935. [Google Scholar]
- Wen, L.; Chen, L.; Sui, Y.X.; Duan, Q.R.; Wei, L.J.; Zhou, Q.; Peng, H.M.; Zhang, Z. Naringenin ameliorates kidney injury by inhibiting TGF-β1/smad signaling pathway in diabetic nephropathy rats. Basic Clin. Med. 2016, 36, 896–901. [Google Scholar]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of glycosidase by ursolic acid: In vitro, in vivo and in silico study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef]
- Ding, H.; Hu, X.; Xu, X.; Zhang, G.; Gong, D. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on alpha-glucosidase. Int. J. Biol. Macromol. 2018, 107, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Ziwei, W.; Na, Z.; Shengbo, F.; Xin, Z. Effect of ursolic acid on obesity-induced insulin resistance in rat liver. Trop. J. Pharm. Res. 2018, 17, 837–842. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef]
- Manna, P.; Ghosh, J.; Das, J.; Sil, P.C. Streptozotocin induced activation of oxidative stress responsive splenic cell signaling pathways: Protective role of arjunolic acid. Toxicol. Appl. Pharm. 2010, 244, 114–129. [Google Scholar] [CrossRef]
- Bacanli, M.; Aydin, S.; Anlar, H.G.; Cal, T.; Undeger Bucurgat, U.; Ari, N.; Basaran, A.A.; Basaran, N. Protective Effects of Ursolic Acid in the Kidneys of Diabetic Rats. Turk. J. Pharm. Sci. 2018, 15, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Kuo, Y.H.; Lin, C.H.; Ho, H.Y.; Shih, C.C. Tormentic acid, a major component of suspension cells of Eriobotrya japonica, suppresses high-fat diet-induced diabetes and hyperlipidemia by glucose transporter 4 and AMP-activated protein kinase phosphorylation. J. Agric. Food Chem. 2014, 62, 10717–10726. [Google Scholar] [CrossRef]
- Manna, P.; Sil, P.C. Impaired redox signaling and mitochondrial uncoupling contributes vascular inflammation and cardiac dysfunction in type 1 diabetes: Protective role of arjunolic acid. Biochimie 2012, 94, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Flávia, A.S.; Julyanne, T.F.; Bruno, R.A.; Tiago, S.M.; Armenio, A.C.A.S.; Gerly, A.C.B.; Mariana, H.C.; Vietla, S.R. Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012, 11, 1–8. [Google Scholar]
- Liu, J.; Wang, X.; Chen, Y.-P.; Mao, L.-F.; Shang, J.; Sun, H.-B.; Zhang, L.-Y. Maslinic acid modulates glycogen metabolism by enhancing the insulin signaling pathway and inhibiting glycogen phosphorylase. Chin. J. Nat. Med. 2014, 12, 259–265. [Google Scholar] [CrossRef]
- Birgani, G.A.; Ahangarpour, A.; Khorsandi, L.; Moghaddam, H.F. Anti-diabetic effect of betulinic acid on streptozotocin-nicotinamide induced diabetic male mouse model. Braz. J. Pharm. Sci. 2018, 54, e17171. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.; Sil, P.C. Arjunolic acid: Beneficial role in type 1 diabetes and its associated organ pathophysiology. Free Radic. Res. 2012, 46, 815–830. [Google Scholar] [CrossRef]
- Ding, H.; Ni, M.; Zhang, G.; Liao, Y.; Hu, X.; Zhang, Y.; Gong, D. The inhibition of oleanolic acid on protein non-enzymatic glycation. LWT 2020, 125, 109253. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Shabani, R.; Mojaddami, S. Preventive effects of betulinic acid on streptozotocin-nicotinamide induced diabetic nephropathy in male mouse. J. Nephropathol. 2016, 5, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.L.; Wang, X.T.; Cheng, Y.; Zhao, J.G.; Zhou, Y.J.; Yang, J.J.; Qi, M.Y. Ursolic acid improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-induced rats. Biomed. Pharm. 2018, 105, 915–921. [Google Scholar] [CrossRef]
- Mkhwanazi, B.N.; Serumula, M.R.; Myburg, R.B.; van Heerden, F.R.; Musabayane, C.T. Antioxidant effects of maslinic acid in livers, hearts and kidneys of streptozotocin-induced diabetic rats: Effects on kidney function. Ren Fail 2014, 36, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Jinping, L.; Xia, L.; Renyong, Y. Ursolic Acid Provides Kidney Protection in Diabetic Rats. Curr. Ther. Res. 2013, 75, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, J.S.; Zhang, X.; Wu, Y.J.; Huang, K.; Zheng, L. Ursolic acid inhibits early lesions of diabetic nephropathy. Int. J. Mol. Med. 2010, 26, 565–570. [Google Scholar] [PubMed] [Green Version]
- Mkhwanazi, B.N.; van Heerden, F.R.; Mavondo, G.A.; Mabandla, M.V.; Musabayane, C.T. Triterpene derivative improves the renal function of streptozotocin-induced diabetic rats: A follow-up study on maslinic acid. Ren Fail 2019, 41, 547–554. [Google Scholar] [CrossRef]
- Wang, X.T.; Gong, Y.; Zhou, B.; Yang, J.J.; Cheng, Y.; Zhao, J.G.; Qi, M.Y. Ursolic acid ameliorates oxidative stress, inflammation and fibrosis in diabetic cardiomyopathy rats. Biomed Pharm. 2018, 97, 1461–1467. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Y.X.; Lv, H.; Jian, T.Y.; Ding, X.Q.; Li, J.W.; Liu, Y.; Ren, B.R. Study on in vitro activities of triterpene acids from leaf of Eriobotrya japonica against diabetes and its complications. J. Plant Resour. Environ. 2020, 29, 78–80. [Google Scholar]
- Xu, L.; Wang, C. Research Progress and Prospect of Oleanolic Acid in Treatment of Diabetes. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 228–234. [Google Scholar]
- Zhang, M.F.; Shen, Y.Q. Research Advances in Pharmacological Effects of oleanolic Acid in HyPoglycemia and Antidiabetic Complications. Anti-Infect. Pharm. 2015, 12, 801–806. [Google Scholar]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell Physiol. 2019, 234, 17212–17231. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, S.; Ou, B.; Liu, Q.; Dai, J.; Ji, C.; Zhou, H.; Huang, H.; Ma, Y. The Role of JNk Signaling Pathway in Obesity-Driven Insulin Resistance. Diabetes Metab. Syndr. Obes. 2020, 13, 1399–1406. [Google Scholar] [CrossRef]
- Dua, T.K.; Joardar, S.; Chakraborty, P.; Bhowmick, S.; Saha, A.; de Feo, V.; Dewanjee, S. Myricitrin, a Glycosyloxyflavone in Myrica esculenta Bark Ameliorates Diabetic Nephropathy via Improving Glycemic Status, Reducing Oxidative Stress, and Suppressing Inflammation. Molecules 2021, 26, 258. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Lan, F.; Xiang, C.; Shi, W.; Xie, Y.; Tao, Z. Clinical observation on treating 63 cases of chronic glomerulonephritis with Shencha. Guangxi J. Tradit. Chin. Med. 2013, 36, 29–31. [Google Scholar]
- Chen, H.; Zhang, M. Structure and Health Effects of Natural Products on Diabetes Mellitus; Springer: Singapore, 2021. [Google Scholar]






| No. | Tested Substances | Study Design and Protocol | Ref. |
|---|---|---|---|
| 1 | 2-Caffeoyl-L-tartaric acid, rosmarinic acid | α-Glucosidase inhibitory activity and molecular docking | [66] |
| 2 | 95% EEF of 80% ethanol extract | Oral glucose tolerance test in normal C57BL/6J mice | [39] |
| α-Glucosidase inhibitory activity | |||
| 3 | Ethanol extract | α-Glucosidase and α-amylase inhibitory activity | [46] |
| 4 | Ethanol extract, aqueous and EtOAc fractions of ethanol extract, 25 compounds isolated from EtOAc fraction | Measurement of pro-inflammation cytokine in vitro | [56] |
| Xylene-induced acute inflammatory model of mice | |||
| 5 | Ethanol extract | α-Glucosidase inhibitory activity | [46] |
| Antioxidant activity (DPPH and FRAP assays) | |||
| 6 | Siphonol B, orthosiphols B, G, I and N | Measurement of 2-NBDG uptake in 3T3-L1 adipocytes | [74] |
| PTP1B inhibitory activity | |||
| 7 | Aqueous extract | Oral glucose tolerance test | [62] |
| Plasma analysis (insulin, cholesterol, GLP-1, and ghrelin levels) in diabetic rats | |||
| 8 | 70% Ethanol extract and 9 fractions | Antioxidant activity (DPPH assay) | [47] |
| 9 | 50% Methanol extract | Antioxidant activity (DPPH, ABTS, iron chelating and FRAP assays) | [49] |
| 10 | Ethanol extract | Pancreatic lipase inhibitory activity in vitro | [40] |
| Biochemical serum analysis (TG, TC, LDL, lipase, and glucose levels) in HFD-induced rats | |||
| Measurement of leptin, adiponectin, insulin, and HOMA-IR index in HFD-induced rats | |||
| Determination of antioxidant activity in liver tissue in HFD-induced rats | |||
| Histological assessment of liver tissues in HFD-induced rats | |||
| 11 | Aqueous extract | Antioxidant activity (DPPH and ABTS assays) | [50] |
| Cytotoxicity assay, embryotoxicity assay | |||
| 12 | Aqueous extract | 1H-NMR spectroscopic analysis of urine of diabetic rats | [63] |
| 13 | Aqueous, 50% ethanol and ethanol extracts | Acute toxicity study in rats | [84] |
| 14 | Clerodens A–D | Assay for inhibitory ability against LPS-induced NO production in RAW264.7 macrophages | [16] |
| 15 | 50% Ethanol extract | Oral carbohydrate challenge tests in normal and diabetic rats (respectively starch, surcose, and glucose loading) | [41] |
| 16 | Hexane fraction of 70% ethanol extract | Glucose stimulated insulin secretion test | [75] |
| Real time-polymerase chain reaction | |||
| 17 | Aqueous extract | Effects on glucose uptake | [78] |
| 18 | Aqueous extract | The developmental toxicity study in pregnant rats | [87] |
| 19 | Sub-fraction 2 of chloroform extract | Determination of blood glucose level in diabetic rats | [34] |
| Measurement of glucose absorption in the everted rat jejunum, measurement of glucose uptake in isolated rat hemi-diaphragms | |||
| 20 | Methanol extract | Antioxidant activity (DPPH assay) | [48] |
| 21 | Aqueous extract | Effects on glucose uptake and glucose consumption | [77] |
| 22 | 50% Ethanol extract and sinensetin | α-Glucosidase and α-amylase inhibitory activity | [68] |
| 23 | Aqueous extract | Antioxidant activity (ABTS and FRAP assays) | [51] |
| Determination of anti-AGEs formation capacity | |||
| 24 | Aqueous extract | Salmonella/microsome mutation assay, mouse bone marrow micronucleus test | [88] |
| 25 | Chloroform extract and its sub-fraction 2 | Subcutaneous glucose tolerance test in normal rats | [42] |
| 26 | 50% Ethanol extract | Acute toxicity study in rats | [86] |
| Subchronic toxicity study in rats | |||
| 27 | Methanol extract | Acute toxicity study in rats | [85] |
| 28 | Aqueous extract | Oral glucose tolerance test and plasma analysis in normal and diabetic rats | [43] |
| 29 | Aqueous, 50% methanol, methanol, 70% acetone and chloroform extracts | Antioxidant activity (DPPH assay) | [52] |
| 30 | Neoorthosiphonone A | Assay for inhibitory ability against LPS-induced NO production in macrophage-like J774.1 cells | [57] |
| 31 | Siphonols A–E | Assay for inhibitory ability against LPS-induced NO production in macrophage-like J774.1 cells | [58] |
| No. | Compounds | Diabetes and Diabetic Complications | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| 1 | Caffeic acid | Diabetes | Lowers blood glucose level | [110] |
| 2 | Ferulic acid | Diabetes | Lowers blood glucose level; lowers the activities of ALT and AST in the serum | [95] |
| Diabetic cardiomyopathy and liver dysfunction | Decreases the content of AGEs in the liver and heart; decreases the number of apoptotic hepatocytes and cardiomyocytes; reduces histological changes in liver tissues; increases the activity of SOD in the liver and heart | |||
| 3 | Methyl caffeate | Diabetes | Lowers blood glucose level; increases hepatic glycogen level; decreases glucose-6-phosphatase activity; increases the size and number of islets; increases GLUT4 expression; improves β-cells | [96] |
| 4 | Protocatechuic acid | Diabetes | Lowers blood glucose level | [102,103] |
| Diabetic nephropathy and liver dysfunction | Decreases lipid hydroperoxides in liver and kidney; decreases TC, TGs, LDL-C and VLDL-C levels and increases HDL-C level in liver and kidney; reduces histological changes in liver and kidney | |||
| 5 | Rosmarinic acid | Diabetes | Reduces blood glucose, TC, TGs and lipid peroxides levels; inhibitors of α-amylase, α-glucosidase, DPP-IV and PTB1B; lowers the formation of MDA and AGEs; reduces the levels of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, NO and nuclear factor kappa-B (NF-κB); increases the activity of SOD; increases the glucose uptake of muscle cells through activation of AMPK phosphorylation; improves insulin sensitivity; increases GLUT4 expression in skeletal muscle; protects pancreatic β-cells | [98,99,100,101,104,106] |
| Diabetic vascular dysfunction | Decreases IL-1β and TNF-αlevels and the expression of endothelin converting enzyme-1; improves structural alterations in the endothelium | [111] | ||
| 6 | Salvianolic acid C | Diabetic cardiomyopathy | Enhances intracellular adenosine triphosphate (ATP) content in the myocardial tissues; reduces ROS, lipid peroxidation and protein carbonylation level in myocardial tissues; improves SOD level in cardiac tissues; reduces histological abnormality | [108] |
| 7 | Vanillic acid | Diabetes | Lowers blood glucose level; decreases the concentration of lipid hydroperoxides | [94] |
| Diabetic nephropathy and liver dysfunction | Increases the activities of antioxidants in kidney and liver; reduces the levels of AST and ALT in liver; decreases the levels of urea, uric acid, and creatinine in kidney; reduces histological changes in liver and renal tissues |
| No. | Compounds | Diabetes and Diabetic Complications | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| 1 | Baicalein | Diabetes | Lowers blood glucose and MDA level; inhibits gluconeogenesis of hepatocytes; decreases the expressions of glucose-6-phosphatase; increase SOD activity; promotes glucose uptake and glycolysis; increases the expression of PI3K and Akt; increase hepatic glycogen level | [112,113,127,128] |
| Diabetic nephropathy | Lowers HOMA-IR level; restores normal renal function; mitigates renal oxidative stress; lowers the level of NF-κB; ameliorates the structural changes in renal tissues; normalizes the levels of serum pro-inflammatory cytokines and liver function enzymes | [122] | ||
| 2 | Isoquercitrin | Diabetes | Lowers blood glucose, serum HOMA-IR, DPP-IV mRNA levels; increases glucose uptake of hepatocytes; increases mRNA expression of Akt and PI3K; increases SOD, HDL-C, insulin and GLP-1 levels; improves pancreatic atrophy and necrosis | [116] |
| Diabetic liver dysfunction | Reduces serum ALT and AST levels; prevents hepatocytes architecture and hepatic necrosis; suppresses apoptosis and promotes regeneration of hepatocytes | |||
| 3 | Naringenin | Diabetes | Lowers blood glucose, MDA and glycosylated hemoglobin levels; lowers the activities of ALT and AST in serum; increases serum insulin levels; increases the expression of GLUT-4; protects the pancreatic tissues in histopathological study; normalizes lipid concentrations in the serum | [114,117,118,119] |
| Diabetic liver dysfunction | Decreases lipid peroxidation level in liver; decreases the number of vacuolated liver cells and degree of vacuolisation | [120] | ||
| Diabetic nephropathy | Decreases the 24 h-urinary protein, kidney index and glomerular area; increases creatinine clearance rate; decreases lipid peroxidation level in kidney tissue; increases the activity of SOD; decreases renal IL-1β, IL-6 and TNF-α levels; lowers NF-κB p65 expression in kidney; improves kidney histology; reduces apoptosis | [120,121,123,129] | ||
| Diabetic retinopathy | Increases levels of neuroprotective factors, tropomyosin related kinase B and synaptophysin in diabetic retina; ameliorates the levels of apoptosis regulatory proteins in diabetic retina | [126] | ||
| 4 | Prunin | Diabetes | Inhibitory activity against PTP1B and α-glucosidase; stimulates glucose uptake; increases the expression of p-Akt and p-PI3K | [115] |
| 5 | Sinensetin | Diabetes | Inhibitory activity on α-glucosidase and α-amylase | [68] |
| No. | Compounds | Diabetes and Diabetic Complications | Effects and Mechanisms | Ref. |
|---|---|---|---|---|
| 1 | Arjunolic acid | Diabetes | Lowers blood glucose, NO, MDA and protein carbonylation levels; increases the activities of antioxidant enzymes; increases cell viability and decreases cell death; reduces pathological lesion; prevents the expression of c-Jun N-terminal kinase (JNK) | [134,137,141] |
| Diabetic cardiomyopathy | Reduces the levels of vascular inflammation markers; increases the activities of the antioxidant enzymes and cellular redox ratio; decreases DNA oxidation in cardiac tissue; reduces histological changes in cardiac tissues; reduces the number of apoptotic cells | |||
| Diabetic liver dysfunction | Reduces the secretion of ALT, the overproduction of ROS and RNS; reduces histological changes in liver tissues; prevents cell death | |||
| Diabetic nephropathy | Reduces kidney weight to body weight ratio, glomerular area, glomerular volume, BUN and creatinine; reduces the activation of NF-κB; prevents cell death; keeps the kidney close to normal physiological state | |||
| 2–3 | α, β-Amyrin | Diabetes | Lowers blood glucose, LDL, VLDL levels; increases insulin levels; protects islets of Langerhans | [138] |
| 4 | Betulinic acid | Diabetes | Lowers blood glucose level; improves insulin sensitivity; decreases insulin resistance by the alternation of some insulin biomakers; improves pancreatic islets diameter and number; improves pancreatic histology | [140] |
| 5 | Euscaphic acid | Diabetes | Inhibitory activity on α-glucosidase and the formation of Amadori, which is an early product of nonenzymatic glycosylation | [150] |
| 6 | Maslinic acid | Diabetes | Increases hepatic glycogen accumulation; inhibits glycogen phosphorylase activity; induces the phosphorylation level of IRβ and Akt | [139] |
| Diabetic nephropathy | Increases the activity of antioxidant enzymes in renal tissues; increases Na+ output, Na+ excretion rates, fractional excretion of Na+; increases glomerular filtration rate; decreases plasma aldosterone and creatinine levels; diminishes the expression of GLUT1 and GLUT2 in diabetic kidney | [145,148] | ||
| 7 | Oleanolic acid | Diabetes | Lowers blood glucose, LDL and free fatty acids levels; increases insulin level; inhibitory activity on α-glucosidase, α-amylase and PIP1B; inhibits the formation of AGEs products; improve insulin tolerance; inhibits gluconeogenesis; increases serum HDL level; decreases levels of IL-1b, IL-6 and TNFα; increases the activity of SOD; improve glycogen level by the increasing expression of Akt and decreasing expression of glucose-6-phosphatase; increases the expression of IR and IRS-1 | [131,133,142,151] |
| Diabetic liver dysfunction | Decreases the levels of IL-1β, IL-6 and TNFα in liver; decreases the expression of NF-κB; decreases ROS production; increases the activity of SOD | [133,152] | ||
| 8 | Tormentic acid | Diabetes | Lowers blood glucose, leptin and total lipids levels; increases the protein contents of phospho-AMPK and GLUT4 in skeletal muscle | [136] |
| Diabetic liver dysfunction | Reduces histological changes in liver tissues; decreases the mRNA level of glucose-6-phosphatase in liver tissues; increases the protein contents of hepatic phospho-AMPK | |||
| 9 | Ursolic acid | Diabetes | Lowers blood glucose, MDA and LDL levels; inhibits α-amylase and α-glucosidase activity; increases SOD activities; decreases TNF-α and IL-1β level; increases liver glycogen level; decreases the expression of PTP-1B protein; increases the expression of IRS-2 protein | [130,132,135] |
| Diabetic cardiomyopathy | Decreases levels of AGEs, TNF-α, IL-1β and ROS; increases the activity of SOD in myocardium | [149] | ||
| Diabetic nephropathy | Lowers the levels of BUN, creatinine and MDA; lowers urine albumin excretion, renal oxidative stress level, NF-κB activity; prevents the expression of JNK; improves renal structural abnormalities | [144,146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, J.; Li, N.; Liu, J.; Zhou, J.; Zhuang, P.; Chen, H. A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications. Molecules 2022, 27, 444. https://doi.org/10.3390/molecules27020444
Wang Q, Wang J, Li N, Liu J, Zhou J, Zhuang P, Chen H. A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications. Molecules. 2022; 27(2):444. https://doi.org/10.3390/molecules27020444
Chicago/Turabian StyleWang, Qirou, Jia Wang, Nannan Li, Junyu Liu, Jingna Zhou, Pengwei Zhuang, and Haixia Chen. 2022. "A Systematic Review of Orthosiphon stamineus Benth. in the Treatment of Diabetes and Its Complications" Molecules 27, no. 2: 444. https://doi.org/10.3390/molecules27020444