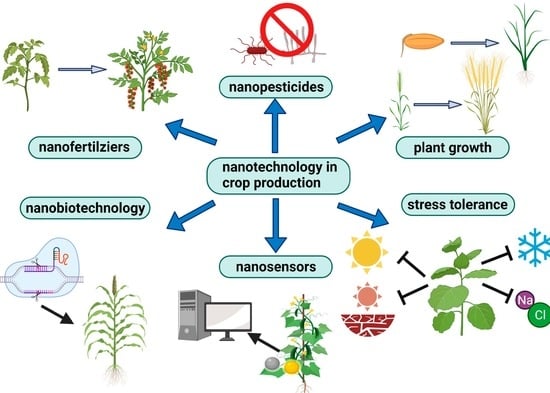
The Applications of Nanotechnology in Crop Production
Abstract
:1. Introduction
2. Nanofertilizers
3. Nanopesticides
4. Nanotechnology in Regulating Seed Germination, and Plant Growth
5. Nanotechnology in Mediating Abiotic Stress Tolerance
6. Nanosensors Used to Monitor Living Plants
7. Nanobiotechnology in Genome Modification
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chem-ical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, V.K.; Manoharan, S.R.R.; Subramanian, S.; Moon, A. Nanotechnology in Spine Surgery: A Current Update and Critical Review of the Literature. World Neurosurg. 2019, 123, 142–155. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; White, J.C.; Wang, Z.; Xing, B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. Health 2018, 6, 77–83. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for Precision and Sustainable Agriculture: Current State and Fu-ture Perspectives. J. Agric. Food Chem. 2018, 66, 6487–6503. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Arya, S.K.; Bhardwaj, N.; Khatri, M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts 2021, 23, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirer, G.S.; Silva, T.N.; Jackson, C.T.; Thomas, J.B.; Ehrhardt, D.W.; Rhee, S.Y.; Mortimer, J.C.; Landry, M.P. Nano-technology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 2021, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, J.P.; Wu, H.; Newkirk, G.M.; Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 2019, 14, 541–553. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Soni, R.; Tripathi, V.; Mishra, A.K.; Kumar, P. Nanotechnological interventions for plant health improvement and sustainable agriculture. 3 Biotech 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Ex-posure to Seeds of Valuable Crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.-H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Wong, M.H.; Lew, T.T.S.; Bisker, G.; Lee, M.A.; Kaplan, A.; Dong, J.; Liu, A.T.; Koman, V.B.; Sinclair, R.; et al. Na-nosensor Technology Applied to Living Plant Systems. Ann. Rev. Anal. Chem. 2017, 10, 113–140. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Ku-marasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Ha, N.M.C.; Nguyen, T.H.; Wang, S.-L.; Nguyen, A.D. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res. Chem. Intermed. 2018, 45, 51–63. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.; Pinto, F.G.; Vergutz, L.; Tronto, J.; da Costa, L.M. Layered Double Hydroxides: New Technology in Phosphate Fertilizers Based on Nanostructured Materials. ACS Sustain. Chem. Eng. 2016, 5, 399–409. [Google Scholar] [CrossRef]
- Azeez, L.; Adejumo, A.L.; Simiat, O.M.; Lateef, A. Influence of calcium nanoparticles (CaNPs) on nutritional qualities, radical scavenging attributes of Moringa oleifera and risk assessments on human health. J. Food Meas. Charact. 2020, 14, 2185–2195. [Google Scholar] [CrossRef]
- Kanjana, D. Foliar application of magnesium oxide nanoparticles on nutrient element concentrations, growth, physiological, and yield parameters of cotton. J. Plant. Nutr. 2020, 43, 3035–3049. [Google Scholar] [CrossRef]
- Hua, K.-H.; Wang, H.-C.; Chung, R.-S.; Hsu, J.-C. Calcium carbonate nanoparticles can enhance plant nutrition and insect pest tolerance. J. Pestic. Sci. 2015, 40, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant. Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total. Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.A.; Prajapati, D.; Bhagat, D.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Chitosan nanofertilizer to foster source activity in maize. Int. J. Biol. Macromol. 2020, 145, 226–234. [Google Scholar] [CrossRef]
- Saini, S.; Kumar, P.; Sharma, N.C.; Sharma, N.; Balachandar, D. Nano-enabled Zn fertilization against conventional Zn ana-logues in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2021, 282, 110016. [Google Scholar] [CrossRef]
- Meier, S.; Moore, F.; Morales, A.; González, M.-E.; Seguel, A.; Meriño-Gergichevich, C.; Rubilar, O.; Cumming, J.; Aponte, H.; Alarcón, D.; et al. Synthesis of calcium borate nanoparticles and its use as a potential foliar fertilizer in lettuce (Lactuca sativa) and zucchini (Cucurbita pepo). Plant. Physiol. Biochem. 2020, 151, 673–680. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2020, 10, 2. [Google Scholar] [CrossRef]
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 2019, 18, 101079. [Google Scholar] [CrossRef]
- Sherkhane, A.S.; Suryawanshi, H.H.; Mundada, P.S.; Shinde, B.P. Control of Bacterial Blight Disease of Pomegranate Using Silver Nanoparticles. J. Nanomed. Nanotechnol. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and assessment of nano-encapsulated bioherbicides based on biopolymers and essential oil. Ind. Crop. Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Sharma, A.; Sharma, M.; Bhalla, N.; Estrela, P.; Jain, A.; Thakur, P.; Thakur, A. Nanomaterial Fungicides: In Vitro and In Vivo Antimycotic Activity of Cobalt and Nickel Nanoferrites on Phytopathogenic Fungi. Glob. Chall. 2017, 1, 1700041. [Google Scholar] [CrossRef] [Green Version]
- Pho, Q.H.; Losic, D.; Ostrikov, K.; Tran, N.N.; Hessel, V. Perspectives on plasma-assisted synthesis of N-doped nanoparticles as nanopesticides for pest control in crops. React. Chem. Eng. 2020, 5, 1374–1396. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelsalam, N.R.; Fouda, M.M.; Mackled, M.I.; Al-Jaddadi, M.A.; Ali, H.M.; Siddiqui, M.H.; Kandil, E.E. Soil Application of Nano Silica on Maize Yield and Its Insecticidal Activity against Some Stored Insects after the Post-Harvest. Nanomaterials 2020, 10, 739. [Google Scholar] [CrossRef]
- Shukla, G.; Gaurav, S.S.; Singh, A. Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs (Holotrichia sp.). Int. Nano Lett. 2020, 10, 131–139. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Das, S.; Yadav, A.; Debnath, N. Entomotoxic efficacy of aluminium oxide, titanium dioxide and zinc oxide nanoparticles against Sitophilus oryzae (L.): A comparative analysis. J. Stored Prod. Res. 2019, 83, 92–96. [Google Scholar] [CrossRef]
- Dweck, H.K.M.; Ebrahim, S.A.M.; Thoma, M.; Mohamed, A.A.M.; Keesey, I.; Trona, F.; Lavista-Llanos, S.; Svatos, A.; Sachse, S.; Knaden, M.; et al. Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, e2829–e2835. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, C.; Greiner, A.; Wendorff, J.H. Design of pheromone releasing nanofibers for plant protection. Polym. Adv. Technol. 2011, 22, 407–413. [Google Scholar] [CrossRef]
- Bhagat, D.; Samanta, S.K.; Bhattacharya, S. Efficient Management of Fruit Pests by Pheromone Nanogels. Sci. Rep. 2013, 3, 1294. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S.; Husen, A. Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res. Lett. 2021, 16, 156. [Google Scholar] [CrossRef]
- Balah, M.A.; Pudake, R.N. Use nanotools for weed control and exploration of weed plants in nanotechnology. In Nanoscience for Sustainable Agriculture; Pudake, R.N., Chauhan, N., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 207–231. [Google Scholar]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Dilbaghi, N.; Sidhu, M.; Kim, K.-H. Development of nanoformulation approaches for the control of weeds. Sci. Total Environ. 2017, 586, 1272–1278. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; da Silva, C.M.G.; Pasquoto, T.; Lima, R.; Fraceto, L.F. Solid Lipid Nanoparticles Co-loaded with Simazine and Atrazine: Preparation, Characterization, and Evaluation of Herbicidal Activity. J. Agric. Food Chem. 2015, 63, 422–432. [Google Scholar] [CrossRef]
- Elmer, W.H.; Ma, C.; White, J.C. Nanoparticles for plant disease management. Curr. Opin. Environ. Sci. Health 2018, 6, 66–70. [Google Scholar] [CrossRef]
- Varympopi, A.; Dimopoulou, A.; Theologidis, I.; Karamanidou, T.; Kerou, A.K.; Vlachou, A.; Karfaridis, D.; Papafotis, D.; Hatzinikolaou, D.G.; Tsouknidas, A.; et al. Bactericides Based on Copper Nanoparticles Restrain Growth of Important Plant Pathogens. Pathogens 2020, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Esyanti, R.R.; Farah, N.; Bajra, B.D.; Nofitasari, D.; Martien, R.; Sunardi, S.; Safitri, R. Comparative Study of Nano-chitosan and Synthetic Bactericide Application on Chili Pepper (Capsicum annuum L.) Infected by Xanthomonas campestris. AGRIVITA J. Agric. Sci. 2020, 42, 11. [Google Scholar] [CrossRef] [Green Version]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Zainol Hilmi, N.H.; Jeffery Daim, L.D. Preparation of Chi-tosan–Hexaconazole Nanoparticles as Fungicide Nanodelivery System for Combating Ganoderma Disease in Oil Palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef] [Green Version]
- Farooq, T.; Adeel, M.; He, Z.; Umar, M.; Shakoor, N.; da Silva, W.; Elmer, W.; White, J.C.; Rui, Y. Nanotechnology and Plant Viruses: An Emerging Disease Management Approach for Resistant Pathogens. ACS Nano 2021, 15, 6030–6037. [Google Scholar] [CrossRef]
- Adeel, M.; Farooq, T.; White, J.C.; Hao, Y.; He, Z.; Rui, Y. Carbon-based nanomaterials suppress tobacco mosaic virus (TMV) infection and induce resistance in Nicotiana benthamiana. J. Hazard. Mater. 2021, 404, 124167. [Google Scholar] [CrossRef]
- El-Dougdoug, N.K.; Bondok, A.M.; El-Dougdoug, K.A. Evaluation of Silver Nanoparticles as Antiviral Agent against ToMV and PVY in Tomato Plants. Middle East. J. Appl. Sci. 2018, 8, 100–111. [Google Scholar]
- Singh, N.; Khandual, A.; Prabhat, G. Preliminary test of functionalized ZnO2 against Bipolaris sorokiniana and other seed associated mycoflora for better wheat germination. Res. J. Biotechnol. 2016, 11, 60–73. [Google Scholar]
- Parveen, A.; Mazhari, B.B.Z.; Rao, S. Impact of bio-nanogold on seed germination and seedling growth in Pennisetum glaucum. Enzyme Microb. Technol. 2016, 95, 107–111. [Google Scholar] [CrossRef]
- Maroufpoor, N.; Mousavi, M.; Hatami, M.; Rasoulnia, A.; Lajayer, B.A. Mechanisms Involved in Stimulatory and Toxicity Effects of Nanomaterials on Seed Germination and Early Seedling Growth. In Advances in Phytonanotechnology; Ghorbanpour, M., Wani, S.H., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 153–181. [Google Scholar]
- Khan, M.A.; Khan, T.; Mashwani, Z.-U.-R.; Riaz, M.S.; Ullah, N.; Ali, H.; Nadhman, A. Plant cell nanomaterials interaction: Growth, physiology and secondary metabolism. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 23–54. [Google Scholar]
- Azimi, R.; Feizi, H.; Hosseini, M.K. Can Bulk and Nanosized Titanium Dioxide Particles Improve Seed Germination Features of Wheatgrass (Agropyron desertorum). Not. Sci. Biol. 2013, 5, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Haghighi, M.; Teixeira da Silva, J.A. The effect of N-TiO2 on tomato, onion, and radish seed germination. J. Crop. Sci. Biotechnol. 2014, 17, 221–227. [Google Scholar] [CrossRef]
- Wan, J.; Wang, R.; Wang, R.; Ju, Q.; Wang, Y.; Xu, J. Comparative Physiological and Transcriptomic Analyses Reveal the Toxic Effects of ZnO Nanoparticles on Plant Growth. Environ. Sci. Technol. 2019, 53, 4235–4244. [Google Scholar] [CrossRef]
- Awasthi, A.; Bansal, S.; Jangir, L.K.; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Effect of ZnO Nanoparticles on Germination of Triticum aestivum Seeds. Macromol. Symp. 2017, 376, 1700043. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanoparticle Res. 2012, 14, 1294. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant. Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of Silver Nanoparticles on Growth of Wheat Under Heat Stress. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Disfani, M.N.; Mikhak, A.; Kassaee, M.Z.; Maghari, A. Effects of nano Fe/SiO2fertilizers on germination and growth of barley and maize. Arch. Agron. Soil Sci. 2017, 63, 817–826. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.-H.; Liu, J.-X.; Feng, K.; Xu, Z.-S.; Xiong, A.-S. Advances in genomic, transcriptomic, proteomic, and metabolomic approaches to study biotic stress in fruit crops. Crit. Rev. Biotechnol. 2019, 39, 680–692. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Gharib, F.A.E.-L.; Ghazi, S.M.; Hegazi, A.Z.; El Hafz, A.G.M.A. Physiological Responses of Two Varieties of Common Bean (Phaseolus vulgaris L.) to Foliar Application of Silver Nanoparticles. Nanomater. Nanotechnol. 2016, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Sarmast, M.K.; Salehi, H. Silver Nanoparticles: An Influential Element in Plant Nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012, 27, 510–517. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant. Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Arif, Y.; Siddiqui, H.; Sami, F.; Zaidi, R.; Azam, A.; Alam, P.; Hayat, S. Nanoparticles enhances the salinity toxicity tolerance in Linum usitatissimum L. by modulating the antioxidative enzymes, photosynthetic efficiency, redox status and cellular damage. Ecotoxicol. Environ. Saf. 2021, 213, 112020. [Google Scholar] [CrossRef] [PubMed]
- Namjoyan, S.; Sorooshzadeh, A.; Rajabi, A.; Aghaalikhani, M. Nano-silicon protects sugar beet plants against water deficit stress by improving the antioxidant systems and compatible solutes. Acta Physiol. Plant. 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Mushinskiy, A.A.; Aminova, E.V.; Korotkova, A.M. Evaluation of tolerance of tubers Solanum tuberosum to silica nanoparticles. Environ. Sci. Pollut. Res. 2018, 25, 34559–34569. [Google Scholar] [CrossRef]
- Kumaraswamy, R.; Saharan, V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Sharma, S.S.; Rakshit, S.; Raliya, R.; Biswas, P. Chitosan-silicon nanofertilizer to enhance plant growth and yield in maize (Zea mays L.). Plant. Physiol. Biochem. 2021, 159, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Sepehri, A. Beneficial Role of MWCNTs and SNP on Growth, Physiological and Photosynthesis Performance of Barley under NaCl Stress. J. Soil Sci. Plant. Nutr. 2018, 18, 752–771. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Liu, Q.; Lin, L.; Li, F.-J.; Han, Y.; Song, Z.-G. Reduction of arsenic toxicity in two rice cultivar seedlings by different nanoparticles. Ecotoxicol. Environ. Saf. 2018, 159, 261–271. [Google Scholar] [CrossRef]
- Gowayed, S.; Al-Zahrani, H.; Metwali, E. Improving the salinity tolerance in potato (Solanum tuberosum) by exogenous application of silicon dioxide nanoparticles. Int. J. Agric. Biol. 2017, 19, 183–192. [Google Scholar]
- Chen, Z.; Wang, Q. Graphene ameliorates saline-alkaline stress-induced damage and improves growth and tolerance in alfalfa (Medicago sativa L.). Plant. Physiol. Biochem. 2021, 163, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef]
- Khan, Z.; Upadhyaya, H. Impact of Nanoparticles on Abiotic Stress Responses in Plants: An Overview. In Nanomaterials in Plants, Algae and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 305–322. [Google Scholar]
- Faiz, S.; Yasin, N.A.; Khan, W.U.; Shah, A.A.; Akram, W.; Ahmad, A.; Ali, A.; Naveed, N.H.; Riaz, L. Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus carota: Modulation in polyamines and antioxidant enzymes. Int. J. Phytoremediat. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D.; González-Morales, S. Chitosan-PVA and Copper Nanoparticles Improve Growth and Overexpress the SOD and JA Genes in Tomato Plants under Salt Stress. Agronomy 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Elsheery, N.I.; Sunoj, V.; Wen, Y.; Zhu, J.; Muralidharan, G.; Cao, K. Foliar application of nanoparticles mitigates the chilling effect on photosynthesis and photoprotection in sugarcane. Plant. Physiol. Biochem. 2020, 149, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: A life cycle study. Environ. Sci. Pollut. Res. 2021, 28, 13712–13724. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, F.; Raja, N.I.; Razzaq, A.; Komatsu, S. Gel-free/label-free proteomic analysis of wheat shoot in stress tolerant varie-ties under iron nanoparticles exposure. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2016, 1864, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Linh, T.M.; Mai, N.C.; Hoe, P.T.; Lien, L.Q.; Ban, N.K.; Hien, L.T.T.; Chau, N.H.; Van, N.T. Metal-Based Nanoparticles Enhance Drought Tolerance in Soybean. J. Nanomater. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Komatsu, S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2015, 128, 280–297. [Google Scholar] [CrossRef]
- Yata, V.K.; Tiwari, B.C.; Ahmad, I. Research Trends and Patents in Nano-food and Agriculture. In Nanoscience in Food and Agriculture 5; Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Zadran, S.; Standley, S.; Wong, K.; Otiniano, E.; Amighi, A.; Baudry, M. Fluorescence resonance energy transfer (FRET)-based biosensors: Visualizing cellular dynamics and bioenergetics. Appl. Microbiol. Biotechnol. 2012, 96, 895–902. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Duyne, R.P.V. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Wujcik, E.K.; Wei, H.; Zhang, X.; Guo, J.; Yan, X.; Sutrave, N.; Wei, S.; Guo, Z. Antibody nanosensors: A detailed review. RSC Adv. 2014, 4, 43725–43745. [Google Scholar] [CrossRef]
- Zhang, J.; Landry, M.P.; Barone, P.W.; Kim, J.-H.; Lin, S.; Ulissi, Z.W.; Lin, D.; Mu, B.; Boghossian, A.A.; Hilmer, A.J.; et al. Molecular recognition using corona phase complexes made of synthetic polymers adsorbed on carbon nanotubes. Nat. Nanotechnol. 2013, 8, 959–968. [Google Scholar] [CrossRef]
- Wang, Z.L. Nanopiezotronics. Adv. Mater. 2007, 19, 889–892. [Google Scholar] [CrossRef]
- Wu, H.; Nißler, R.; Morris, V.; Herrmann, N.; Hu, P.; Jeon, S.-J.; Kruss, S.; Giraldo, J.P. Monitoring Plant Health with Near-Infrared Fluorescent H2O2 Nanosensors. Nano Lett. 2020, 20, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Ast, C.; Schmälzlin, E.; Löhmannsröben, H.-G.; Van Dongen, J.T. Optical Oxygen Micro- and Nanosensors for Plant Applications. Sensors 2012, 12, 7015–7032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, K.; Chang, Y.F.; Horikawa, K.; Hatsugai, N.; Higuchi, Y.; Hashida, M.; Yoshida, Y.; Matsuda, T.; Arai, Y.; Nagai, T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat. Commun. 2012, 3, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, Y.; Yoshimura, M.; Sato, Y.; Kuwata, K.; Toh, S.; Holbrook-Smith, D.; Zhang, H.; McCourt, P.; Itami, K.; Kinoshita, T.; et al. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 2015, 349, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Alova, A.; Erofeev, A.; Gorelkin, P.; Bibikova, T.; Korchev, Y.; Majouga, A.; Bulychev, A. Prolonged oxygen depletion in microwounded cells of Chara corallina detected with novel oxygen nanosensors. J. Exp. Bot. 2019, 71, 386–398. [Google Scholar] [CrossRef]
- Giraldo, J.P.; Landry, M.P.; Kwak, S.-Y.; Jain, R.M.; Wong, M.H.; Iverson, N.M.; Ben-Naim, M.; Strano, M.S. A Ratiometric Sensor Using Single Chirality Near-Infrared Fluorescent Carbon Nanotubes: Application to In Vivo Monitoring. Small 2015, 11, 3973–3984. [Google Scholar] [CrossRef] [Green Version]
- Krebs, M.; Held, K.; Binder, A.; Hashimoto, K.; Den Herder, G.; Parniske, M.; Kudla, J.; Schumacher, K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012, 69, 181–192. [Google Scholar] [CrossRef]
- Cho, J.-H.; Swanson, C.J.; Chen, J.; Li, A.; Lippert, L.G.; Boye, S.E.; Rose, K.; Sivaramakrishnan, S.; Chuong, C.-M.; Chow, R.H. The GCaMP-R Family of Genetically Encoded Ratiometric Calcium Indicators. ACS Chem. Biol. 2017, 12, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Prole, D.L.; Shen, Y.; Lin, Z.; Gnanasekaran, A.; Liu, Y.; Chen, L.; Zhou, H.; Chen, S.R.W.; Usachev, Y.M.; et al. Red fluorescent genetically encoded Ca2+ indicators for use in mitochondria and endoplasmic reticulum. Biochem. J. 2014, 464, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Son, D.; Park, S.Y.; Kim, B.; Koh, J.T.; Kim, T.H.; An, S.; Jang, D.; Kim, G.T.; Jhe, W.; Hong, S. Nanoneedle Transistor-Based Sensors for the Selective Detection of Intracellular Calcium Ions. ACS Nano 2011, 5, 3888–3895. [Google Scholar] [CrossRef]
- Jones, A.M.; Danielson, J.Å.; Manojkumar, S.N.; Lanquar, V.; Grossmann, G.; Frommer, W.B. Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. Elife 2014, 3, e01741. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, A.; Champion, A.; Legrand, J.; Lavenus, J.; Mast, D.B.; Brunoud, G.; Oh, J.; Guyomarc, H.S.; Pizot, M.; Farmer, E.E.; et al. A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat. Commun. 2015, 6, 6043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, Q.; Wang, Y.; Oh, J.; Jin, S.; Park, Y.; Zhou, T.; Zhao, B.; Ruan, W.; Jung, Y.M. A reagent-assisted method in SERS detection of methyl salicylate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective Detection of Ethylene Gas Using Carbon Nanotube-based Devices: Utility in Determination of Fruit Ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef]
- Dalal, A.; Rana, J.S.; Kumar, A. Ultrasensitive Nanosensor for Detection of Malic Acid in Tomato as Fruit Ripening Indicator. Food Anal. Methods 2017, 10, 3680–3686. [Google Scholar] [CrossRef]
- Gültekin, A.; Karanfil, G.; Kuş, M.; Sonmezoglu, S.; Say, R. Preparation of MIP-based QCM nanosensor for detection of caffeic acid. Talanta 2014, 119, 533–537. [Google Scholar] [CrossRef]
- Chaudhuri, B.; Hörmann, F.; Frommer, W.B. Dynamic imaging of glucose flux impedance using FRET sensors in wild-type Arabidopsis plants. J. Exp. Bot. 2011, 62, 2411–2417. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, B.; Hörmann, F.; Lalonde, S.; Brady, S.M.; Orlando, D.A.; Benfey, P.; Frommer, W.B. Protonophore- and pH-insensitive glucose and sucrose accumulation detected by FRET nanosensors in Arabidopsis root tips. Plant J. 2008, 56, 948–962. [Google Scholar] [CrossRef] [Green Version]
- Chai, Y.; Chen, C.; Luo, X.; Zhan, S.; Kim, J.; Luo, J.; Wang, X.; Hu, Z.; Ying, Y.; Liu, X. Cohabiting Plant-Wearable Sensor In Situ Monitors Water Transport in Plant. Adv. Sci. 2021, 8, 2003642. [Google Scholar] [CrossRef]
- Kim, J.J.; Allison, L.K.; Andrew, T.L. Vapor-printed polymer electrodes for long-term, on-demand health monitoring. Sci. Adv. 2019, 5, eaaw0463. [Google Scholar] [CrossRef] [Green Version]
- Maity, D.; Kumar, R.T.R. Polyaniline Anchored MWCNTs on Fabric for High Performance Wearable Ammonia Sensor. ACS Sens. 2018, 3, 1822–1830. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Kumar, S.; Jasrotia, P.; Singh, D.P.; Singh, G.P. Nanosensors for Plant Disease Diagnosis: Current Understand-ing and Future Perspectives. In Nanoscience for Sustainable Agriculture; Pudake, R.N., Chauhan, N., Kole, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 189–205. [Google Scholar]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K. Nanodiagnostics for plant pathogens. Environ. Chem. Lett. 2017, 15, 7–13. [Google Scholar] [CrossRef]
- Li, Z.; Yu, T.; Paul, R.; Fan, J.; Yang, Y.; Wei, Q. Agricultural nanodiagnostics for plant diseases: Recent advances and challenges. Nanoscale Adv. 2020, 2, 3083–3094. [Google Scholar] [CrossRef]
- Lau, H.Y.; Wang, Y.; Wee, E.J.H.; Botella, J.R.; Trau, M. Field Demonstration of a Multiplexed Point-of-Care Diagnostic Platform for Plant Pathogens. Anal. Chem. 2016, 88, 8074–8081. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, T.R.; Salleh, M.A.M.; Sijam, K.; Rahim, R.A.; Mohsenifar, A.; Safarnejad, R.; Tabatabaei, M. Fluorometric immunoassay for detecting the plant virus Citrus tristeza using carbon nanoparticles acting as quenchers and antibodies labeled with CdTe quantum dots. Microchim. Acta 2016, 183, 2277–2287. [Google Scholar] [CrossRef]
- Fang, Y.; Umasankar, Y.; Ramasamy, R.P. Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analytics 2014, 139, 3804–3810. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Park, M.; Wang, Y.; Gordiichuk, P.; Yeap, W.C.; Rais, S.K.M.; Kulaveerasingam, H.; Strano, M.S. Nanocarriers for Transgene Expression in Pollen as a Plant Biotechnology Tool. ACS Mater. Lett. 2020, 2, 1057–1066. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated Delivery of siRNA to Intact Plant Cells for Efficient Gene Knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.-J.; et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef]




| NPs | Type | Original Pesticide | Concentration | Target | Reference |
|---|---|---|---|---|---|
| Chitosan | Insecticide | Spinosad/permethrin | 10 mg/L | Drosophila melanogaster | [27] |
| Zinc oxide (ZnO) | Insecticide | Aspergillus niger | 20 mg/L | Holotrichia sp. | [34] |
| Aluminium oxide (Al2O3) | Insecticide | — | 2 g/kg | Sitophilus oryzae L. | [36] |
| Nanogel | Insecticide | Methyl eugenol | 12 mg/mL | Bactrocera dorsalis | [39] |
| Nanogel | Herbicide | Savory essential oil | 15 mL/L | weeds | [29] |
| Polysaccharide | Herbicide | Metsulfuron methyl | 0.5 g/L | Chenopodium album | [43] |
| Solid lipid | Herbicide | Atrazine and simazine | 0.3 kg/ha | Raphanus raphanistrum | [44] |
| Silver (Ag) | Bactericide | Leaf extracts (holy basil) | 15 mM | Xanthomonas axonopodis pv. punicae | [28] |
| Copper (Cu) | Bactericide | — | 240 mg/L | Agrobacterium tumefaciens, Dickeya dadantii, Erwinia amylovora, Pectobacterium carotovorum and Pseudomonas savastanoi pv. Savastanoi | [46] |
| Chitosan | Bactericide | Streptomycin sulfate | 1 mg/mL | Xanthomonas campestris | [47] |
| Cobalt ferrite (CoFe2O4) and Nickel ferrite (NiFe2O4) | Fungicide | — | 500 mg/L | Fusarium oxysporum, Colletotrichum gloeosporioides and Dematophora necatrix | [31] |
| Chitosan | Fungicide | Hexaconazole | 10 ug/L | Ganoderma boninense | [49] |
| Cu | Fungicide | — | 0.5 mg/mL | Fusarium solani and Fusarium oxysporum | [30] |
| Carbon nanotubes (CNTs) | Antiviral-pesticide | — | 200 mg/L | Tobacco mosaic virus (TMV) | [51] |
| Ag | Antiviral-pesticide | — | 50 mg/L | Tomato mosaic virus (ToMV) and Potato virus Y (PVY) | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhou, H.; Zhou, J. The Applications of Nanotechnology in Crop Production. Molecules 2021, 26, 7070. https://doi.org/10.3390/molecules26237070
Liu C, Zhou H, Zhou J. The Applications of Nanotechnology in Crop Production. Molecules. 2021; 26(23):7070. https://doi.org/10.3390/molecules26237070
Chicago/Turabian StyleLiu, Chenxu, Hui Zhou, and Jie Zhou. 2021. "The Applications of Nanotechnology in Crop Production" Molecules 26, no. 23: 7070. https://doi.org/10.3390/molecules26237070