Dental Applications of Carbon Nanotubes
Abstract
:1. Introduction
2. Carbon Nanotubes in Dentistry
2.1. Characteristics of Carbon Nanotubes
2.2. Single-Wall and Multiple-Wall Carbon Nanotubes
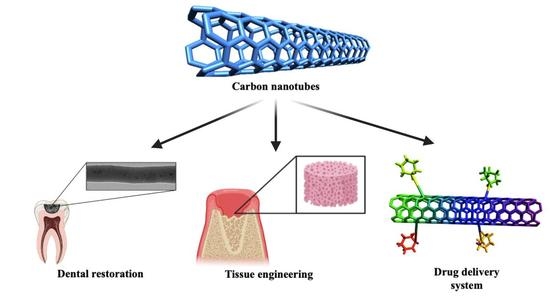
2.3. Applications of Carbon Nanotubes in Dentistry
3. Dental Restorative Materials
3.1. Characteristics of Glass Ionomer Cements
3.2. Benefits and Applications of Glass Ionomers in Dental Restorations
3.3. Major Drawbacks of Using Glass Ionomers
3.4. Nanotechnology in Glass Ionomers
4. Carbon Nanotubes in Guided Bone Regeneration (GBR)
4.1. Mechanical Properties of Nanofiber Polymeric Membranes Reinforced with Carbon Nanotubes
4.2. Effect of Carbon Nanotubes on Cells
4.3. Preparation of Polymeric Membranes Reinforced with Carbon Nanotubes
5. Carbon Nanotubes in Drug Delivery Systems
6. Conclusions and Future Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrar, R.; Arman, H.; Mousa, S. The Fourth Industrial Revolution (Industry 4.0): A social innovation perspective. Technol. Innov. Manag. Rev. 2017, 7, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Maynard, A.D. Navigating the Fourth Industrial Revolution. Nat. Nanotechnol. 2015, 10, 1005–1006. [Google Scholar] [CrossRef]
- AlKahtani, R.N. The implications and applications of nanotechnology in dentistry: A review. Saudi Dent. J. 2018, 30, 107–116. [Google Scholar] [CrossRef]
- Schleyer, T.L. Nanodentistry. Fact or fiction? J. Am. Dent. Assoc. 2000, 131, 1567–1568. [Google Scholar] [CrossRef]
- Kanaparthy, R.; Kanaparthy, A. The changing face of dentistry: Nanotechnology. Int. J. Nanomed. 2011, 6, 2799–2804. [Google Scholar] [CrossRef] [Green Version]
- Mok, Z.H.; Proctor, G.; Thanou, M. Emerging nanomaterials for dental treatments. Emerg. Top. Life Sci. 2021, 4, 613–625. [Google Scholar] [CrossRef]
- Ge, J.; Cui, F.Z.; Wang, X.M.; Feng, H.L. Property variations in the prism and the organic sheath within enamel by nanoindentation. Biomaterials 2005, 26, 3333–3339. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwon, H.K.; Kim, B.I. Effect of dentinal tubule occlusion by dentifrice containing nano-carbonate apatite. J. Oral Rehabil. 2008, 35, 847–853. [Google Scholar] [CrossRef]
- Ozak, S.T.; Ozkan, P. Nanotechnology and dentistry. Eur. J. Dent. 2013, 7, 145–151. [Google Scholar] [CrossRef]
- Freitas, R.A. Molecular robots and other high-tech possibilities. J. Am. Dent. Assoc. 2000, 131, 1559–1565. [Google Scholar] [CrossRef]
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef]
- Journet, C.; Bernard, C.; Lyon, U.; Bernier, P. Production of carbon nanotubes. Artic. Appl. Phys. A 1998. [Google Scholar] [CrossRef]
- Gao, Z.; Varela, J.A.; Groc, L.; Lounis, B.; Cognet, L. Toward the suppression of cellular toxicity from single-walled carbon nanotubes. Biomater. Sci. 2016, 4, 230–244. [Google Scholar] [CrossRef] [Green Version]
- Kleverlaan, C.J.; Van Duinen, R.N.B.; Feilzer, A.J. Mechanical properties of glass ionomer cements affected by curing methods. Dent. Mater. 2004, 20, 45–50. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in dentistry: State of the art and future challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical reinforcement of polymers using carbon nanotubes. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of carbon nanotubes in biotechnology and biomedicine. J. Biomed. Nanotechnol. 2006, 1, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Maniecki, T.; Shtyka, O.; Mierczynski, P.; Ciesielski, R.; Czylkowska, A.; Leyko, J.; Mitukiewicz, G.; Dubkov, S.; Gromov, D. Carbon nanotubes: Properties, synthesis, and application. Fibre Chem. 2018, 50, 297–300. [Google Scholar] [CrossRef]
- Kechagioglou, P.; Andriotis, E.; Papagerakis, P.; Papagerakis, S. Multiwalled carbon nanotubes for dental applications. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1922, pp. 121–128. [Google Scholar]
- McIntyre, R.A. Common nano-materials and their use in real world applications. Sci. Prog. 2012, 95, 1–22. [Google Scholar] [CrossRef]
- Andrews, R.; Weisenberger, M.C. Carbon nanotube polymer composites. Curr. Opin. Solid State Mater. Sci. 2004, 8, 31–37. [Google Scholar] [CrossRef]
- Garchitorena, M.I.; Garchitorena, M.I. Vidrios bioactivos en odontología restauradora. Odontoestomatologia 2019, 21, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Imazato, S.; Kohno, T.; Tsuboi, R.; Thongthai, P.; Xu, H.H.K.; Kitagawa, H. Cutting-edge filler technologies to release bio-active components for restorative and preventive dentistry. Dent. Mater. J. 2020, 39, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials—Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, B.; Treasure, E.; Dummer, P.; Dunstan, F.; Gilmour, A.; Jones, R.; Phillips, C.; Stevens, J.; Rees, J.; Richmond, S. Challenges with studies investigating longevity of dental restorations—A critique of a systematic review. J. Dent. 2001, 29, 155–161. [Google Scholar] [CrossRef]
- Tyas, M.J.; Anusavice, K.J.; Frencken, J.E.; Mount, G.J. Minimal intervention dentistry—A review: FDI Commission Project 1-97. Int. Dent. J. 2000, 50, 1–12. [Google Scholar] [CrossRef]
- Xie, D.; Weng, Y.; Guo, X.; Zhao, J.; Gregory, R.L.; Zheng, C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent. Mater. 2011, 27, 487–496. [Google Scholar] [CrossRef]
- Poole, S.F.; Pitondo-Silva, A.; Oliveira-Silva, M.; Moris, I.C.M.; Gomes, E.A. Influence of different ceramic materials and surface treatments on the adhesion of Prevotella intermedia. J. Mech. Behav. Biomed. Mater. 2020, 111, 104010. [Google Scholar] [CrossRef]
- Fan, C.; Chu, L.; Rawls, H.R.; Norling, B.K.; Cardenas, H.L.; Whang, K. Development of an antimicrobial resin-a pilot study. Dent. Mater. 2011, 27, 322–328. [Google Scholar] [CrossRef]
- Matis, B.A.; Cochran, M.J.; Carlson, T.J.; Guba, C.; Eckert, G.J. A three-year clinical evaluation of two dentin bonding agents. J. Am. Dent. Assoc. 2004, 135, 451–457. [Google Scholar] [CrossRef]
- Rekow, E.D.; Bayne, S.C.; Carvalho, R.M.; Steele, J.G. What constitutes an ideal dental restorative material? Adv. Dent. Res. 2013, 25, 18–23. [Google Scholar] [CrossRef]
- Raadsheer, M.C.; Van Eijden, T.M.G.J.; Van Ginkel, F.C.; Prahl-Andersen, B. Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. J. Dent. Res. 1999, 78, 31–42. [Google Scholar] [CrossRef]
- Khan, A.S.; Khan, M.; Rehman, I.U. Nanoparticles, properties, and applications in glass ionomer cements. In Nanobiomaterials in Clinical Dentistry; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 93–108. ISBN 9781455731275. [Google Scholar]
- Ramesh, B.; Kulvinder, K.W.; Aseem, P.; Anil, C. Dental amalgam: An update. Compend. Contin. Educ. Dent. 2010, 13, 204–208. [Google Scholar] [CrossRef]
- Lynch, C.D.; Frazier, K.B.; McConnell, R.J.; Blum, I.R.; Wilson, N.H.F. State-of-the-art techniques in operative dentistry: Contemporary teaching of posterior composites in UK and Irish dental schools. Br. Dent. J. 2010, 3, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.J.; Burke, F.J.; Watson, D.J.; Owen, F.J. Materials for restoration of primary teeth: I. Conventional materials and early glass ionomers. Dent. Update 2001, 28, 486–491. [Google Scholar] [CrossRef]
- Gracis, S.; Thompson, V.; Ferencz, J.; Silva, N.; Bonfante, E. A new classification system for all-ceramic and ceramic-like restorative materials. Int. J. Prosthodont. 2015, 28, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Marghalani, H.Y. Resin-based dental composite materials. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Berlin, Germany, 2016; pp. 357–405. [Google Scholar] [CrossRef]
- Gallo, M.; Abouelleil, H.; Chenal, J.M.; Adrien, J.; Lachambre, J.; Colon, P.; Maire, E. Polymerization shrinkage of resin-based composites for dental restorations: A digital volume correlation study. Dent. Mater. 2019, 35, 1654–1664. [Google Scholar] [CrossRef]
- Opdam, N.J.M.; Van De Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.D.N.J.M.; Van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Flores-Cedillo, M.L.; Alvarado-Estrada, K.N.; Pozos-Guillén, A.J.; Murguía-Ibarra, J.S.; Vidal, M.A.; Cervantes-Uc, J.M.; Rosales-Ibáñez, R.; Cauich-Rodríguez, J.V. Multiwall carbon nanotubes/polycaprolactone scaffolds seeded with human dental pulp stem cells for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2016, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Browning, W.D. The benefits of glass ionomer self-adhesive materials in restorative dentistry. Compend. Contin. Educ. Dent. 2006, 27, 308–314. [Google Scholar]
- Croll, T.P.; Nicholson, J.W. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatric Dent. 2002, 24, 423–429. [Google Scholar]
- Zhao, J.; Xie, D. A novel hyperbranched poly(acrylic acid) for improved resin-modified glass-ionomer restoratives. Dent. Mater. 2011, 27, 478–486. [Google Scholar] [CrossRef]
- Tarasingh, P.; Sharada Reddy, J.; Suhasini, K.; Hemachandrika, I. Comparative evaluation of antimicrobial efficacy of resin-modified glass ionomers, compomers and giomers—An in vitro study. J. Clin. Diagn. Res. 2015, 9, ZC85–ZC87. [Google Scholar] [CrossRef]
- Williams, J.A.; Billington, R.W.; Pearson, G.J. The comparative strengths of commercial glass-ionomer cements with and without metal additions. Br. Dent. J. 1992, 172, 279–282. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Pek, Y.S.; Cheang, P. Physico-mechanical properties of a fast-set highly viscous GIC restorative. J. Oral Rehabil. 2003, 30, 1–8. [Google Scholar] [CrossRef]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Du, W.; Zhou, X.-D.; Yu, H.-Y. Review of research on the mechanical properties of the human tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, J.W.; Sidhu, S.K.; Czarnecka, B. Enhancing the Mechanical Properties of Glass-Ionomer Dental Cements: A Review. Materials 2020, 13, 2510. [Google Scholar] [CrossRef] [PubMed]
- Tüzüner, T.; Dimkov, A.; Nicholson, J.W. The effect of antimicrobial additives on the properties of dental glass-ionomer cements: A review. Acta Biomater. Odontol. Scand. 2019, 5, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garoushi, S.; Vallittu, P.; Lassila, L. Hollow glass fibers in reinforcing glass ionomer cements. Dent. Mater. 2017, 33, e86–e93. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K. Glass-ionomer cement restorative materials: A sticky subject? Aust. Dent. J. 2011, 56, 23–30. [Google Scholar] [CrossRef]
- Baig, M.S.; Fleming, G.J.P. Conventional glass-ionomer materials: A review of the developments in glass powder, polyacid liquid and the strategies of reinforcement. J. Dent. 2015, 43, 897–912. [Google Scholar] [CrossRef]
- Knight, G.M. The benefits and limitations of glass-ionomer cements and their use in contemporary dentistry. In Glass-Ionomers in Dentistry; Springer: Cham, Switzerland, 2016; pp. 57–79. ISBN 978-3-319-22625-5. [Google Scholar]
- Sidhu, S.K.; Watson, T.F. Resin-modified glass-ionomer materials. Part 1: Properties. Dent. Update 1995, 22, 429–432. [Google Scholar] [PubMed]
- Kerezoudi, C.; Samanidou, V.F.; Palaghias, G. Nanobiomaterials in restorative dentistry. In Nanobiomaterials in Dentistry: Applications of Nanobiomaterials; Elsevier Inc.: Oxford, UK, 2016; Volume 11, pp. 107–132. ISBN 9780323428903. [Google Scholar]
- Moheet, I.A.; Luddin, N.; Ab Rahman, I.; Masudi, S.M.; Kannan, T.P.; Abd Ghani, N.R.N. Evaluation of mechanical properties and bond strength of nano-hydroxyapatite-silica added glass ionomer cement. Ceram. Int. 2018, 44, 9899–9906. [Google Scholar] [CrossRef]
- Thampi, V.A.; Prabhu, M.; Kavitha, K.; Manivasakan, P.; Prabu, P.; Rajendran, V.; Shankar, S.; Kulandaivelu, P. Hydroxyapatite, alumina/zirconia, and nanobioactive glass cement for tooth-restoring applications. Ceram. Int. 2014, 40, 14355–14365. [Google Scholar] [CrossRef]
- Goyal, M.; Sharma, K. Novel multi-walled carbon nanotube reinforced glass-ionomer cements for dental restorations. Mater. Today Proc. 2020, 37, 3035–3037. [Google Scholar] [CrossRef]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Pani, S.C.; Aljammaz, M.T.; Alrugi, A.M.; Aljumaah, A.M.; Alkahtani, Y.M.; Alkhuraif, A. Color stability of glass ionomer cement after reinforced with two different nanoparticles. Int. J. Dent. 2020. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- de Moura, N.K.; Martins, E.F.; Oliveira, R.L.M.S.; de Brito Siqueira, I.A.W.; Machado, J.P.B.; Esposito, E.; Amaral, S.S.; de Vasconcellos, L.M.R.; Passador, F.R.; de Sousa Trichês, E. Synergistic effect of adding bioglass and carbon nanotubes on poly(lactic acid) porous membranes for guided bone regeneration. Mater. Sci. Eng. C 2020, 117, 111327. [Google Scholar] [CrossRef]
- Taton, T.A. Boning up on biology. Nature 2001, 412, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef]
- Teh, S.J.; Lai, C.W. Carbon nanotubes for dental implants. In Applications of Nanocomposite Materials in Dentistry; Elsevier: Duxford, UK, 2018; pp. 93–105. ISBN 9780128137598. [Google Scholar]
- Gholami, F.; Noor, A.F.M. Hydroxyapatite reinforced with multi-walled carbon nanotubes and bovine serum albumin for bone substitute applications. AIP Conf. Proc. 2016, 1791, 20045. [Google Scholar] [CrossRef] [Green Version]
- Pourakbar Saffar, K.; Sudak, L.J.; Federico, S. A biomechanical evaluation of CNT-grown bone. J. Biomed. Mater. Res. Part A 2016, 104, 465–475. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Making carbon nanotubes biocompatible and biodegradable. Chem. Commun. 2011, 47, 10182–10188. [Google Scholar] [CrossRef] [PubMed]
- Miura, J.; Maeda, Y.; Nakai, H.; Zako, M. Multiscale analysis of stress distribution in teeth under applied forces. Dent. Mater. 2009, 25, 67–73. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Seong, W.J. Carbon nanotube-based materials-preparation, biocompatibility, and applications in dentistry. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–76. ISBN 9780128158869. [Google Scholar]
- Sagadevan, S.; Periasamy, M. Recent trends in nanobiosensors and their applications—A review. Rev. Adv. Mater. Sci. 2014, 36, 62–69. [Google Scholar]
- Hahn, B.D.; Lee, J.M.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Lee, B.K.; Shin, D.S.; Kim, H.E. Mechanical and in vitro biological performances of hydroxyapatite-carbon nanotube composite coatings deposited on Ti by aerosol deposition. Acta Biomater. 2009, 5, 3205–3214. [Google Scholar] [CrossRef]
- Marrs, B.; Andrews, R.; Rantell, T.; Pienkowski, D. Augmentation of acrylic bone cement with multiwall carbon nanotubes. J. Biomed. Mater. Res. Part A 2006, 77, 269–276. [Google Scholar] [CrossRef]
- Imazato, S.; Kitagawa, H.; Tsuboi, R.; Kitagawa, R.; Thongthai, P.; Sasaki, J. Non-biodegradable polymer particles for drug delivery: A new technology for “bio-active” restorative materials. Dent. Mater. J. 2017, 36, 524–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf. B Biointerfaces 2012, 93, 226–234. [Google Scholar] [CrossRef]
- Kim, J.J.; Bae, W.J.; Kim, J.M.; Kim, J.J.; Lee, E.J.; Kim, H.W.; Kim, E.C. Mineralized polycaprolactone nanofibrous matrix for odontogenesis of human dental pulp cells. J. Biomater. Appl. 2014, 28, 1069–1078. [Google Scholar] [CrossRef]
- Stutz, C.; Strub, M.; Clauss, F.; Huck, O.; Schulz, G.; Gegout, H.; Benkirane-Jessel, N.; Bornert, F.; Kuchler-Bopp, S. A new polycaprolactone-based biomembrane functionalized with BMP-2 and stem cells improves maxillary bone regeneration. Nanomaterials 2020, 10, 1774. [Google Scholar] [CrossRef]
- Ketabi, M.A.; Shahnavazi, M.; Fekrazad, R.; Tondnevis, F.; Keshvari, H.; Raz, M.; Sadeghi, A.; Abolhasani, M.M. Synthesis and in vitro characterization of carbon nano tube-polycaprolactone composite scaffold for odontoblast cell interaction. In Key Engineering Materials; Trans Tech Publications Ltd.: Zurich, Switzerland, 2017; Volume 720, pp. 114–119. [Google Scholar]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial effects of carbon nanotubes: Size does matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Kim, T.H.; Mandakhbayar, N.; Singh, R.K.; Jang, J.H.; Lee, J.H.; Kim, H.W. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses. Acta Biomater. 2020, 108, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.M.; Yilgor, I.; Yilgor, E.; Erman, B. Electrospinning of polyurethane fibers. Polymer 2002, 43, 3303–3309. [Google Scholar] [CrossRef]
- Uyar, T.; Çökeliler, D.; Doʇan, M.; Koçum, I.C.; Karatay, O.; Denkbaş, E.B. Electrospun nanofiber reinforcement of dental composites with electromagnetic alignment approach. Mater. Sci. Eng. C 2016, 62, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.L.S.; Tribst, J.P.M.; Dal Piva, A.M.O.; Souza, A.C.O. In vitro evaluation of multi-walled carbon nanotube reinforced nanofibers composites for dental application. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1015–1022. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Lagaron, J.M.; Hoa, S.V. Effect of addition of carbon nanofibers and carbon nanotubes on properties of thermoplastic biopolymers. Compos. Sci. Technol. 2010, 70, 1095–1105. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Sun, L.; Aifantis, K.E.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Resin composites reinforced by nanoscaled fibers or tubes for dental regeneration. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Singh, B.G.P.; Baburao, C.; Pispati, V.; Pathipati, H.; Muthy, N.; Prassana, S.R.V.; Rathode, B.G. Carbon nanotubes. A novel drug delivery system. Int. J. Res. Pharm. Chem. 2012, 2, 523–532. [Google Scholar]
- Hilder, T.A.; Hill, J.M. Modeling the loading and unloading of drugs into nanotubes. Small 2009, 5, 300–308. [Google Scholar] [CrossRef]
- Jin, H.; Heller, D.A.; Sharma, R.; Strano, M.S. Size-dependent cellular uptake and expulsion of single-walled carbon nanotubes: Single particle tracking and a generic uptake model for nanoparticles. ACS Nano 2009, 3, 149–158. [Google Scholar] [CrossRef] [PubMed]




| Classification | Characteristics and Use | Reference |
|---|---|---|
| Type I | Luting cements with low fill thickness and rapid setting. Used for the cementation of inlays, crowns, fixed partial dentures, and orthodontic appliances. | [43] |
| Type II | Restorations with particles larger than Type I. | [43] |
| Type II-1 | Considered as esthetic cements available for conventional and resin-modified presentations. | [43] |
| Type II-2 | Reinforced cement for esthetic applications. | [43] |
| Type III | Lining cements and fissure sealants with low viscosity and rapid setting. | [43] |
| Based on composition | Derived from an organic acid and a glass component referred to as acid-base reaction cements. | [44] |
| Resin-modified GICs | Contains an ion-leachable glass, a water-soluble polymeric acid, an organic monomer, and an initiator system. | [45] |
| Polyacid-modified composite resin | Light-polymerized composite resin restoratives with ion-leachable glass particles and an anhydrous polyalkenoic acid. | [46] |
| Metal-reinforced GICs | Mixture of a conventional powder with the addition of a range of metallic powders, such as silver alloys, gold, palladium, and titanium dioxide. | [47] |
| High-viscosity GICs | Have a high powder–liquid ratio and fast setting properties. | [48] |
| Zirconia-reinforced GICs | Contain zirconium oxide, glass powder, tartaric acid (1–10%), polyacrylic acid (20–50%), and deionized water. | [49] |
| Tooth Tissue | Property | Value |
|---|---|---|
| Hardness | 2.0–3.5 GPa * | |
| Enamel | Young’s modulus | 80–120 GPa |
| Fracture toughness | 0.67–3.93 MPa m1/2 | |
| Hardness | 0.3–0.7 GPa | |
| Dentin | Young´s modulus | 10–40 GPa |
| Fracture toughness | 1.1–2.3 MPa m1/2 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Rojas, M.A.; Vega-Cantu, Y.I.; Cordell, G.A.; Rodriguez-Garcia, A. Dental Applications of Carbon Nanotubes. Molecules 2021, 26, 4423. https://doi.org/10.3390/molecules26154423
Castro-Rojas MA, Vega-Cantu YI, Cordell GA, Rodriguez-Garcia A. Dental Applications of Carbon Nanotubes. Molecules. 2021; 26(15):4423. https://doi.org/10.3390/molecules26154423
Chicago/Turabian StyleCastro-Rojas, Marco A., Yadira I. Vega-Cantu, Geoffrey A. Cordell, and Aida Rodriguez-Garcia. 2021. "Dental Applications of Carbon Nanotubes" Molecules 26, no. 15: 4423. https://doi.org/10.3390/molecules26154423