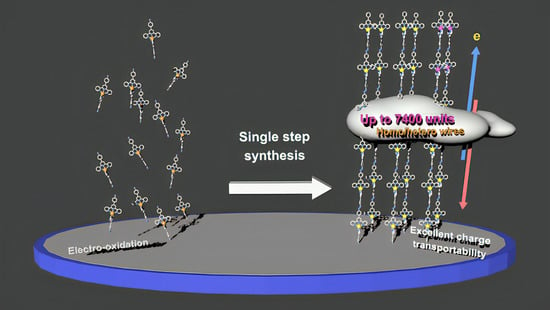
Ultralong π-Conjugated Bis(terpyridine)metal Polymer Wires Covalently Bound to a Carbon Electrode: Fast Redox Conduction and Redox Diode Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrosynthesis of Polymer Wires
2.2. Structural Characterization and Electropolymerization Mechanism
2.3. Electrochemical Performance and Morphology of Electropolymerized Films
2.4. Charge Transport in Long Fe(tpy)2 Polymer Wires
2.5. Hetero-Metal-Complex Wires
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- De Ruiter, G.; van der Boom, M.E. Surface-Confined Assemblies and Polymers for Molecular Logic. Acc. Chem. Res. 2011, 44, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Szaciłowski, K. Digital Information Processing in Molecular Systems. Chem. Rev. 2008, 108, 3481–3548. [Google Scholar] [CrossRef]
- De Ruiter, G.; Tartakovsky, E.; Oded, N.; van der Boom, M.E. Sequential Logic Operations with Surface-Confined Polypyridyl Complexes Displaying Molecular Random Access Memory Features. Angew. Chem. Int. Ed. 2010, 49, 169–172. [Google Scholar] [CrossRef]
- De Silva, A.P. A layer of logic. Nature 2008, 454, 417–418. [Google Scholar] [CrossRef]
- Gupta, T.; van der Boom, M.E. Redox-Active Monolayers as a Versatile Platform for Integrating Boolean Logic Gates. Angew. Chem. Int. Ed. 2008, 47, 5322–5326. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.P.; Uchiyama, S. Molecular logic and computing. Nat. Nanotechnol. 2007, 2, 399–410. [Google Scholar] [CrossRef]
- De Silva, A.P.; James, M.R.; McKinney, B.O.; Pears, D.A.; Weir, S.M. Molecular computational elements encode large populations of small objects. Nat. Mater. 2006, 5, 787–789. [Google Scholar] [CrossRef]
- Margulies, D.; Melman, G.; Shanzer, A. Fluorescein as a model molecular calculator with reset capability. Nat. Mater. 2005, 4, 768–771. [Google Scholar] [CrossRef]
- Lo, W.-Y.; Zhang, N.; Cai, Z.; Li, L.; Yu, L. Beyond Molecular Wires: Design Molecular Electronic Functions Based on Dipolar Effect. Acc. Chem. Res. 2016, 49, 1852–1863. [Google Scholar] [CrossRef]
- Sakamoto, R.; Wu, K.-H.; Matsuoka, R.; Maeda, H.; Nishihara, H. π-Conjugated bis(terpyridine)metal complex molecular wires. Chem. Soc. Rev. 2015, 44, 7698–7714. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Katagiri, S.; Maeda, H.; Nishihara, H. Bis(terpyridine) metal complex wires: Excellent long-range electron transfer ability and controllable intrawire redox conduction on silicon electrode. Coord. Chem. Rev. 2013, 257, 1493–1506. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as Molecular Electronic Components of Functional Devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.J.; Zhang, Y.; Murphy, C.B.; Angell, S.E.; Parker, M.F.; Flynn, B.R.; Jones, W.E. Fluorescent conjugated polymer molecular wire chemosensors for transition metal ion recognition and signaling. Coord. Chem. Rev. 2009, 253, 410–422. [Google Scholar] [CrossRef]
- Nishihara, H.; Kanaizuka, K.; Nishimori, Y.; Yamanoi, Y. Construction of Redox-and Photo-functional Molecular Systems on Electrode Surface for Application to Molecular Devices. Coord. Chem. Rev. 2007, 251, 2674–2687. [Google Scholar] [CrossRef]
- Robertson, N.; McGowan, C.A. A comparison of potential molecular wires as components for molecular electronics. Chem. Soc. Rev. 2003, 32, 96–103. [Google Scholar] [CrossRef]
- Swager, T.M. The Molecular Wire Approach to Sensory Signal Amplification. Acc. Chem. Res. 1998, 31, 201. [Google Scholar] [CrossRef]
- Terasaki, N.; Yamamoto, N.; Hiraga, T.; Yamanoi, Y.; Yonezawa, T.; Nishihara, H.; Ohmori, T.; Sakai, M.; Fujii, M.; Tohri, A.; et al. Plugging a Molecular Wire into Photosystem I: Reconstitution of the Photoelectric Conversion System on a Gold Electrode. Angew. Chem. Int. Ed. 2009, 48, 1585–1587. [Google Scholar] [CrossRef]
- Nitzan, A.; Ratner, M.A. Electron transport in molecular wire junctions. Science 2003, 300, 1384–1389. [Google Scholar] [CrossRef]
- Tuccitto, N.; Ferri, V.; Cavazzini, M.; Quici, S.; Zhavnerko, G.; Licciardello, A.; Rampi, M.A. Highly conductive approximately 40-nm-long molecular wires assembled by stepwise incorporation of metal centres. Nat. Mater. 2009, 8, 41–46. [Google Scholar] [CrossRef]
- Terao, J.; Tanaka, Y.; Tsuda, S.; Kambe, N.; Taniguchi, M.; Kawai, T.; Saeki, A.; Seki, S. Insulated Molecular Wire with Highly Conductive π-Conjugated Polymer Core. J. Am. Chem. Soc. 2009, 131, 18046–18047. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, B.; Frisbie, C.D. Electrical resistance of long conjugated molecular wires. Science 2008, 320, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, A.; Osuka, A. Fully Conjugated Porphyrin Tapes with Electronic Absorption Bands That Reach into Infrared. Science 2001, 293, 79–82. [Google Scholar] [CrossRef]
- Aratani, N.; Osuka, A.; Kim, Y.H.; Jeong, D.H.; Kim, D. Extremely Long, Discrete meso–meso-Coupled Porphyrin Arrays. Angew. Chem. Int. Ed. 2000, 39, 1458–1462. [Google Scholar] [CrossRef]
- Martin, R.E.; Mäder, T.; Diederich, F. Monodisperse Poly(triacetylene) Rods: Synthesis of a 11.9 nm Long Molecular Wire and Direct Determination of the Effective Conjugation Length by UV/Vis and Raman Spectroscopies. Angew. Chem. Int. Ed. 1999, 38, 817–821. [Google Scholar] [CrossRef]
- Davis, W.B.; Svec, W.A.; Ratner, M.A.; Wasielewski, M.R. Molecular-wire behaviour in p-phenylenevinylene oligomers. Nature 1998, 396, 60–63. [Google Scholar] [CrossRef]
- Magoga, M.; Joachim, C. Conductance and transparence of long molecular wires. Phys. Rev. B 1997, 56, 4722. [Google Scholar] [CrossRef]
- Schumm, J.S.; Pearson, D.L.; Tour, J.M. Iterative Divergent/Convergent Approach to Linear Conjugated Oligomers by Successive Doubling of the Molecular Length: A Rapid Route to a 128Å-Long Potential Molecular Wire. Angew. Chem. Int. Ed. 1994, 33, 1360–1363. [Google Scholar] [CrossRef]
- Shah, A.; Adhikari, B.; Martic, S.; Munir, A.; Shahzad, S.; Ahmad, K.; Kraatz, H.B. Electron transfer in peptides. Chem. Soc. Rev. 2015, 44, 1015–1027. [Google Scholar] [CrossRef]
- Joachim, C.; Gimzewski, J.K.; Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 2000, 408, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Ouyang, L.; Lund, A.; Moth-Poulsen, K.; Hamedi, M.M. From Single Molecules to Thin Film Electronics, Nanofibers, e-Textiles and Power Cables: Bridging Length Scales with Organic Semiconductors. Adv. Mater. 2019, 31, 1807286. [Google Scholar] [CrossRef] [PubMed]
- Aragones, A.C.; Martin-Rodriguez, A.; Aravena, D.; Puigmarti-Luis, J.; Amabilino, D.B.; Aliaga-Alcalde, N.; Gonzalez-Campo, A.; Ruiz, E.; Diez-Perez, I. Tuning single-molecule conductance in metalloporphyrin-based wires via supramolecular interactions. Angew. Chem. Int. Ed. 2020, 59, 19193–19201. [Google Scholar] [CrossRef]
- Bryce, M.R. A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. J. Mater. Chem. C 2021, in press. [Google Scholar] [CrossRef]
- Sakamoto, R.; Katagiri, S.; Maeda, H.; Nishimori, Y.; Miyashita, S.; Nishihara, H. Electron Transport Dynamics in Redox-Molecule-Terminated Branched Oligomer Wires on Au(111). J. Am. Chem. Soc. 2015, 137, 734–741. [Google Scholar] [CrossRef]
- Yamanoi, Y.; Sendo, J.; Kobayashi, T.; Maeda, H.; Yabusaki, Y.; Miyachi, M.; Sakamoto, R.; Nishihara, H. A New Method to Generate Arene-Terminated Si(111) & Ge(111) Surfaces via a Palladium-Catalyzed Arylation Reaction. J. Am. Chem. Soc. 2012, 134, 20433–20439. [Google Scholar]
- Nishimori, Y.; Maeda, H.; Katagiri, S.; Sendo, J.; Miyachi, M.; Sakamoto, R.; Yamanoi, Y.; Nishihara, H. Synthesis and Electron Transfer Properties of Metal Complex Oligomer Wires with an Inherent Potential Gradient on Gold Electrode. Macromol. Symp. 2012, 317–318, 276–285. [Google Scholar] [CrossRef]
- Maeda, H.; Sakamoto, R.; Nishimori, Y.; Sendo, J.; Toshimitsu, F.; Yamanoi, Y.; Nishihara, H. Bottom-Up Fabrication of Redox-Active Metal Complex Oligomer Wires on an H-terminated Si(111) Surface. Chem. Commun. 2011, 47, 8644–8646. [Google Scholar] [CrossRef]
- Kurita, T.; Nishimori, Y.; Toshimitsu, F.; Muratsugu, S.; Kume, S.; Nishihara, H. Surface Junction Effects on the Electron Conduction of Molecular Wires. J. Am. Chem. Soc. 2010, 132, 4524–4525. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, Y.; Kanaizuka, K.; Kurita, T.; Nagatsu, T.; Segawa, Y.; Toshimitsu, F.; Muratsugu, S.; Utsuno, M.; Kume, S.; Murata, M.; et al. Superior Electron-Transport Ability of π-Conjugated Redox Molecular Wires Prepared by the Stepwise Coordination Method on a Surface. Chem. Asian J. 2009, 4, 1361–1367. [Google Scholar] [CrossRef]
- Nishimori, Y.; Kanaizuka, K.; Murata, M.; Nishihara, H. Synthesis of Molecular Wires of Linear and Branched Bis(terpyridine) Complex Oligomers and Electrochemical Observation of Through-bond Redox Conduction. Chem. Asian J. 2007, 2, 367–376. [Google Scholar] [CrossRef]
- Kanaizuka, K.; Murata, M.; Nishimori, Y.; Mori, I.; Nishio, K.; Masuda, H.; Nishihara, H. Stepwise Preparation of Linear π-Conjugated Bis(terpyridine)metal Polymer Chains at Gold Surface. Chem. Lett. 2005, 34, 534–535. [Google Scholar] [CrossRef]
- Javey, A.; Kong, J. Carbon Nanotube Electronics; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Wang, C.; Takei, K.; Takahashi, T.; Javey, A. Carbon nanotube electronics—Moving forward. Chem. Soc. Rev. 2013, 42, 2592–2609. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2009, 110, 132–145. [Google Scholar] [CrossRef]
- Wu, J.; Pisula, W.; Müllen, K. Graphenes as Potential Material for Electronics. Chem. Rev. 2007, 107, 718–747. [Google Scholar] [CrossRef] [PubMed]
- Mustafar, S.; Wu, K.-H.; Toyoda, R.; Takada, K.; Maeda, H.; Miyachi, M.; Sakamoto, R.; Nishihara, H. Electrochemical fabrication of one-dimensional porphyrinic wires on electrodes. Inorg. Chem. Front. 2016, 3, 370–375. [Google Scholar] [CrossRef]
- Gremlich, H.-U. Infrared and Raman Spectroscopy. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, N. Copper-Catalyzed Aerobic Oxidative Dehydrogenative Coupling of Anilines Leading to Aromatic Azo Compounds using Dioxygen as an Oxidant. Angew. Chem. Int. Ed. 2010, 49, 6174–6177. [Google Scholar] [CrossRef]
- Kádár, M.; Nagy, Z.; Karancsi, T. The electrochemical oxidation of 4-chloroaniline, 2,4-dichloroaniline and 2,4,6-trichloroaniline in acetonitrile solution. Electrochim. Acta 2001, 46, 1297–1306. [Google Scholar] [CrossRef]
- Adenier, A.; Chehimi, M.M.; Gallardo, I.; Pinson, J. Electrochemical oxidation of aliphatic amines and their attachment to carbon and metal surfaces. Langmuir 2004, 20, 8243–8253. [Google Scholar] [CrossRef]
- Deinhammer, R.S.; Ho, M.J.; Anderegg, W.; Porte, M.D. Electrochemical oxidation of amine-containing compounds: A route to the surface modification of glassy carbon electrodes. Langmuir 1994, 10, 1306–1313. [Google Scholar] [CrossRef]
- Yang, H.-T.; Liang, X.-C.; Wang, Y.-H.; Yang, Y.; Sun, X.-Q.; Miao, C.-B. CuCl2-Mediated Reaction of [60]Fullerene with Amines in the Presence or Absence of Dimethyl Acetylenedicarboxylate: Preparation of Fulleropyrroline or Aziridinofullerene Derivatives. J. Org. Chem. 2013, 78, 11992–11998. [Google Scholar] [CrossRef]
- Miles, M.-H.; Kellett, P.M. Electrochemical Oxidation of Fuels in Liquid Ammonia. J. Electrochem. Soc. 1968, 115, 1225. [Google Scholar] [CrossRef]
- Collin, J.P.; Jouaiti, A.; Sauvage, J.P. Anodic electropolymerization of films of polypyrrole functionalized with metal terpyridyl redox centres. J. Electroanal. Chem. 1990, 286, 75–87. [Google Scholar] [CrossRef]
- Taft, K.L.; Caneschi, A.; Pence, L.E.; Delfs, C.D.; Papaefthymiou, G.C.; Lippard, S.J. Iron and manganese alkoxide cubes. J. Am. Chem. Soc. 1993, 115, 11753–11766. [Google Scholar] [CrossRef]
- Denisevich, P.; Abruna, H.D.; Leidner, C.R.; Meyer, T.J.; Murray, R.W. Electropolymerization of vinylpyridine and vinylbipyridine complexes of iron and ruthenium: Homopolymers, copolymers, reactive polymers. Inorg. Chem. 1982, 21, 2153–2161. [Google Scholar] [CrossRef]
- Aoki, A.; Heller, A. Electron diffusion coefficients in hydrogels formed of cross-linked redox polymers. J. Phys. Chem. 1993, 97, 11014–11019. [Google Scholar] [CrossRef]
- Shigehara, K.; Oyama, N.; Anson, F.C. Electrochemical responses of electrodes coated with redox polymers. Evidence for control of charge-transfer rates across polymeric layers by electron exchange between incorporated redox sites. J. Am. Chem. Soc. 1981, 103, 2552–2558. [Google Scholar] [CrossRef]
- Daum, P.; Lenhard, J.R.; Rolison, D.; Murray, R.W. Diffusional charge transport through ultrathin films of radiofrequency plasma polymerized vinylferrocene at low temperature. J. Am. Chem. Soc. 1980, 102, 4649–4653. [Google Scholar] [CrossRef]
- Daum, P.; Murray, R.W. Charge-transfer diffusion rates and activity relationships during oxidation and reduction of plasma-polymerized vinylferrocene films. J. Phys. Chem. 1981, 85, 389–396. [Google Scholar] [CrossRef]
- Zhang, N.; Lo, W.-Y.; Jose, A.; Cai, Z.; Li, L.; Yu, L. A single-molecular AND gate operated with two orthogonal switching mechanisms. Adv. Mater. 2017, 29, 1701248. [Google Scholar] [CrossRef]
- De Ruiter, G.; Lahav, M.; Keisar, H.; van der Boom, M.E. Sequence-Dependent Assembly to Control Molecular Interface Properties. Angew. Chem. Int. Ed. 2013, 52, 704–709. [Google Scholar] [CrossRef]
- De Ruiter, G.; Lahav, M.; Evmenenko, G.; Dutta, P.; Cristaldi, D.A.; Gulino, A.; van der Boom, M.E. Composite Molecular Assemblies: Nanoscale Structural Control and Spectroelectrochemical Diversity. J. Am. Chem. Soc. 2013, 135, 16533–16544. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-H.; Sakamoto, R.; Maeda, H.; Phua, E.J.H.; Nishihara, H. Ultralong π-Conjugated Bis(terpyridine)metal Polymer Wires Covalently Bound to a Carbon Electrode: Fast Redox Conduction and Redox Diode Characteristics. Molecules 2021, 26, 4267. https://doi.org/10.3390/molecules26144267
Wu K-H, Sakamoto R, Maeda H, Phua EJH, Nishihara H. Ultralong π-Conjugated Bis(terpyridine)metal Polymer Wires Covalently Bound to a Carbon Electrode: Fast Redox Conduction and Redox Diode Characteristics. Molecules. 2021; 26(14):4267. https://doi.org/10.3390/molecules26144267
Chicago/Turabian StyleWu, Kuo-Hui, Ryota Sakamoto, Hiroaki Maeda, Eunice Jia Han Phua, and Hiroshi Nishihara. 2021. "Ultralong π-Conjugated Bis(terpyridine)metal Polymer Wires Covalently Bound to a Carbon Electrode: Fast Redox Conduction and Redox Diode Characteristics" Molecules 26, no. 14: 4267. https://doi.org/10.3390/molecules26144267