Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Pectin/Polyacrylamide Hydrogel
2.2.2. Determination of Gel Fraction
2.2.3. FT-IR Spectroscopic Analysis
2.2.4. X-ray Diffraction
2.2.5. Differential Scanning Calorimetry (DSC)
2.2.6. Morphological Analysis
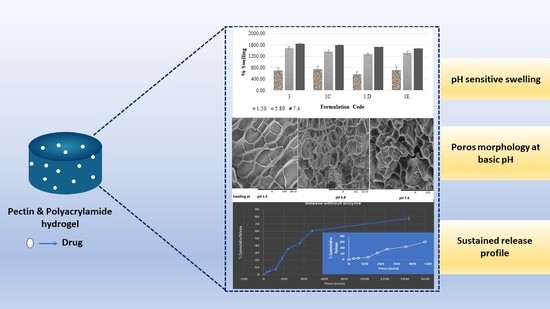
2.2.7. Swelling Studies
2.2.8. Drug Loading
2.2.9. In-Vitro Drug Release Study
3. Results and Discussion
3.1. Synthesis of Hydrogels
3.1.1. Optimization of Pectin/Acrylamide Ratio
3.1.2. Optimization of MBA/KPS Ratio
3.2. Characterization of Budesonide Loaded Optimized Hydrogel
3.2.1. FT-IR Spectroscopic Analysis
3.2.2. X-ray Diffraction
3.2.3. Differential Scanning Calorimetry (DSC)
3.2.4. Morphological Analysis
3.2.5. Drug Loading and In-Vitro Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample availability
References
- Danese, S.; Fiocchi, C. Ulcerative Colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Bressler, B.; Marshall, J.K.; Bernstein, C.N.; Bitton, A.; Jones, J.; Leontiadis, G.I.; Panaccione, R.; Steinhart, A.H.; Tse, F.; Feagan, B.; et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: The Toronto consensus. Gastroenterology 2015, 148, 1035–1058. [Google Scholar] [CrossRef] [Green Version]
- Kornbluth, A.; Sachar, D. Erratum: Ulcerative Colitis Practice Guidelines in Adults: American College of Gastroenterology, Practice Parameters Committee. Am. J. Gastroenterol. 2010, 105, 500. [Google Scholar] [CrossRef]
- Seibold, F.; Fournier, N.; Beglinger, C.; Mottet, C.; Pittet, V.; Rogler, G.; Swiss IBD Cohort Study Group. Topical therapy is underused in patients with ulcerative colitis. J. Crohn’s Colitis 2014, 8, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdalla, M.I.; Herfarth, H. Budesonide for the treatment of ulcerative colitis. Expert Opin. Pharmacother. 2016, 17, 1549–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danese, S.; Hart, A.; Dignass, A.; Louis, E.; D’Haens, G.; Dotan, I.; Rogler, G.; D’Agay, L.; Iannacone, C.; Peyrin-Biroulet, L. Effectiveness of budesonide MMX (Cortiment) for the treatment of mild-to-moderate active ulcerative colitis: Study protocol for a prospective multicentre observational cohort study. BMJ Open Gastroenterol. 2016, 3, e000092. [Google Scholar] [CrossRef] [PubMed]
- Karolewska-Bochenek, K.; Dziekiewicz, M.; Banaszkiewicz, A. Budesonide MMX in Paediatric Patients with Ulcerative Colitis. J. Crohn’s Colitis 2017, 11, 1402. [Google Scholar] [CrossRef]
- Lautenschläger, C.; Schmidt, C.; Fischer, D.; Stallmach, A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014, 71, 58–76. [Google Scholar] [CrossRef]
- Drug Approval Package (Budesonide). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021949_budesonide_toc.cfm (accessed on 20 August 2020).
- Sherlock, M.E.; MacDonald, J.K.; Griffiths, A.M.; Steinhart, A.H.; Seow, C.H. Oral budesonide for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Danese, S.; D’Haens, G.; Moro, L.; Jones, R.; Bagin, R.; Huang, M.; David Ballard, E.; Masure, J.; Travis, S. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: Pooled analysis of two phase 3 studies. Aliment. Pharmacol. Ther. 2015, 41, 409–418. [Google Scholar] [CrossRef]
- Lichtenstein, G.R. Budesonide Multi-matrix for the Treatment of Patients with Ulcerative Colitis. Dig. Dis. Sci. 2016, 61, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-W.; Naeem, M.; Cao, J.; Choi, M.; Kim, W.; Moon, H.R.; Lee, B.L.; Kim, M.-S.; Jung, Y. Enhanced therapeutic efficacy of budesonide in experimental colitis with enzyme/pH dual-sensitive polymeric nanoparticles. Int. J. Nanomed. 2015, 10, 4565–4580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for Colon Targeted Drug Delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chourasia, M.K.; Jain, S.K. Design and Development of Multiparticulate System for Targeted Drug Delivery to Colon. Drug Deliv. 2004, 11, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Philip, A.; Philip, B. Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches. Oman Med. J. 2010, 25, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.; Kost, J.; Hicks, K. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H. Budesonide-Loaded Guar Gum Microspheres for Colon Delivery: Preparation, Characterization and In Vitro/In Vivo Evaluation. Int. J. Mol. Sci. 2015, 16, 2693–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Zhang, W.; Mahuta, K.M.; Mikulski, B.A.; Harvestine, J.N.; Crouse, J.Z.; Lee, J.C.; Kaltchev, M.G.; Tritt, C.S. Novel pectin-based carriers for colonic drug delivery. Pharm. Dev. Technol. 2016, 21, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Emami, J.; Tavakoli, N.; Minaiyan, M.; Rahmani, N.; Dorkoosh, F.; Mahzouni, P. Pectin Film Coated Pellets for Colon-Targeted Delivery of Budesonide: In-Vitro/In-Vivo Evaluation in Induced Ulcerative Colitis in Rat. Iran. J. Pharm. Res. IJPR 2012, 11, 733–745. [Google Scholar]
- Prasad, S.; Aeri, V. Approaches for Targeted Drug Delivery to Colon. Int. J. Drug Deliv. Technol. 2010, 3, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Fei, L.; Cui, F.; Tang, C.; Yin, C. Superporous hydrogels containing poly(acrylic acid-co-acrylamide)/O-carboxymethyl chitosan interpenetrating polymer networks. Biomaterials 2007, 28, 1258–1266. [Google Scholar] [CrossRef]
- Halib, N.; Amin, M.C.I.M.; Ahmad, I. Unique stimuli responsive characteristics of electron beam synthesized bacterial cellulose/acrylic acid composite. J. Appl. Polym. Sci. 2010, 116, 2920–2929. [Google Scholar] [CrossRef]
- Sadeghi, M.; Heidari, B. Crosslinked Graft Copolymer of Methacrylic Acid and Gelatin as a Novel Hydrogel with pH-Responsiveness Properties. Materials 2011, 4, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.; Selvanathan, V.; Sonsudin, F.; Abouloula, C. Erratum: pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. Polymers 2017, 9, 225. [Google Scholar] [CrossRef] [Green Version]









| Formulation Code for Hydrogel | AM (g) | PC (g) | Ratio of MBA/KPS | Gel Fraction of Hydrogel (%) | SD |
|---|---|---|---|---|---|
| 1A | 1.5 | 0.5 | 50/50 | 58.9 | 0.63 |
| 1B | 1.5 | 1 | 50/50 | 49.01 | 2.17 |
| F3 | 2.1 | 0.5 | 50/50 | 77.23 | 1.57 |
| 1C | 2.1 | 1 | 50/50 | 72.29 | 0.53 |
| 1D | 2.8 | 0.5 | 50/50 | 81.08 | 2.47 |
| 1E | 2.8 | 1 | 50/50 | 76.09 | 1.25 |
| Formulation Code for Hydrogel | Ratio of AM/PC | MBA (mg) | KPS (mg) | Gel Fraction of Hydrogel (%) | SD |
|---|---|---|---|---|---|
| F1 | 2.1/0.5 | 10 | 50 | 60.23 | 1.03 |
| F2 | 2.1/0.5 | 20 | 50 | 62.81 | 0.98 |
| F3 | 2.1/0.5 | 50 | 50 | 77.23 | 1.57 |
| F4 | 2.1/0.5 | 70 | 50 | 78.05 | 0.86 |
| F5 | 2.1/0.5 | 90 | 50 | 79.47 | 1.73 |
| F6 | 2.1/0.5 | 50 | 20 | 63.4 | 0.78 |
| F7 | 2.1/0.5 | 50 | 70 | 79.13 | 1.18 |
| Formulation | Zero-Order (R2) | First-Order (R2) | Higuchi (R2) | Hixon-Crowell (R2) | Korsmeyer-Peppas (n) |
|---|---|---|---|---|---|
| Drug loaded hydrogel (F3) | 0.9772 | 0.8720 | 0.9320 | 0.9390 | 0.697 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, M.; Choudhury, H.; D/O Segar Singh, S.K.; Chetty Annan, N.; Bhattamisra, S.K.; Gorain, B.; Mohd Amin, M.C.I. Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics. Molecules 2021, 26, 2704. https://doi.org/10.3390/molecules26092704
Pandey M, Choudhury H, D/O Segar Singh SK, Chetty Annan N, Bhattamisra SK, Gorain B, Mohd Amin MCI. Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics. Molecules. 2021; 26(9):2704. https://doi.org/10.3390/molecules26092704
Chicago/Turabian StylePandey, Manisha, Hira Choudhury, Sahleni Kaur D/O Segar Singh, Naveenya Chetty Annan, Subrat Kumar Bhattamisra, Bapi Gorain, and Mohd Cairul Iqbal Mohd Amin. 2021. "Budesonide-Loaded Pectin/Polyacrylamide Hydrogel for Sustained Delivery: Fabrication, Characterization and In Vitro Release Kinetics" Molecules 26, no. 9: 2704. https://doi.org/10.3390/molecules26092704