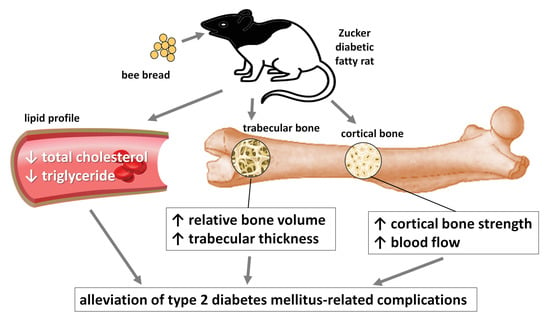
Bee Bread Can Alleviate Lipid Abnormalities and Impaired Bone Morphology in Obese Zucker Diabetic Rats
Abstract
:1. Introduction
2. Results
2.1. Biochemical Analysis
2.2. Macroscopical Analysis of Bones
2.3. Morphological 3D and 2D Analyses of Bones
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Sample Preparation
4.3. Animals
4.4. Biochemical Analysis
4.5. Macroscopical Analysis of Bones
4.6. Morphological 3D and 2D Analyses of Bones
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Milibari, A.A.; Matuure, E.Y.; Gadah, E.M. Prevalence, Determinants and Prevention of Type 2 Diabetes Mellitus (T2DM) in Arabic Countries: A Systematic Review Study. Health Sci. J. 2020, 14. [Google Scholar] [CrossRef]
- Al Mansour, M.A. The Prevalence and Risk Factors of Type 2 Diabetes Mellitus (DMT2) in a Semi-Urban Saudi Population. Int. J. Environ. Res. Public Health 2019, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2009, 204, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Aron, R.A.C.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Ghodsi, M.; Larijani, B.; Keshtkar, A.A.; Nasli-Esfahani, E.; Alatab, S.; Mohajeri-Tehrani, M.R. Mechanisms involved in altered bone metabolism in diabetes: A narrative review. J. Diabetes Metab. Disord. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, C.; Liu, J. The Effects of Type 1 vs. Type 2 Diabetes on Bone Metabolism. World J. Surg. Surg. Res. 2020, 3, 5. [Google Scholar]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotiotics 2020, 9, 811. [Google Scholar] [CrossRef] [PubMed]
- Yucel, B.; Topal, E.; Kosoglu, M. Bee Products as Functional Food. In Superfood and Functional Food An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017; p. 21. [Google Scholar]
- Sidor, E.; Dżugan, M. Drone Brood Homogenate as Natural Remedy for Treating Health Care Problem: A Scientific and Practical Approach. Molecules 2020, 25, 5699. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Eckholm, B.J.; Huang, M.H. A comparison of bee bread made by Africanized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie 2012, 44, 52–63. [Google Scholar] [CrossRef]
- Ivanišová, E.; Kačániová, M.; Frančáková, H.; Petrová, J.; Hutková, J.; Brovarskyi, V.; Velychko, S.; Adamchuk, L.; Schubertová, Z.; Musilová, J. Bee bread - perspective source of bioactive compounds for future. Potravinarstvo Slovak J. Food Sci. 2015, 9, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Barene, I.; Daberte, I.; Siksna, S. Investigation of bee bread and development of its dosage forms. Med. Teor. ir Prakt. 2014, 21, 16–22. [Google Scholar] [CrossRef]
- Habryka, C.; Kruczek, M.; Drygaś, B. Bee Products Used in Apitherapy. World Sci. News 2016, 48, 121–125. [Google Scholar]
- Urcan, A.C.; Al Marghitas, L.; Dezmirean, D.S.; Bobis, O.; Bonta, V.; I Muresan, C.; Margaoan, R. Chemical Composition and Biological Activities of Beebread—Review. Bull. Univ. Agric. Sci. Veter- Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2017, 74, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, G.S.; Veening-Griffioen, D.H.; Boon, W.P.; Hooijmans, C.R.; Moors, E.H.; Schellekens, H.; Van Meer, P.J. Comparison of drug efficacy in two animal models of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Pharmacol. 2020, 879, 173153. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules 2019, 24, 4394. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s Disease: A Focus on the Effects of Propolis and Royal Jelly. Oxidative Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Prnova, M.S.; Svik, K.; Slovak, L.; Kisska, P.; Kovacik, A.; Brindza, J. Consumption of bee bread influences glycaemia and development of diabetes in obese spontaneous diabetic rats. Biologia 2020, 75, 705–711. [Google Scholar] [CrossRef]
- Shishehbor, F.; Azemi, M.E.; Zameni, D.; Saki, A. Inhibitory Effect of Hydroalcoholic Extracts of Barberry, Sour Cherry and Cornelian Cherry on α-Amylase and α-Glucosidase Activities. Int. J. Pharm. Res. Allied Sci. 2016, 5, 423–428. [Google Scholar]
- Tavdidishvili, D.; Khutsidze, T.; Pkhakadze, M.; Vanidze, M.; Kalandia, A. Flavonoids in Georgian Bee Bread and Bee Pollen. J. Chem. Chem. Eng. 2014, 8, 676–681. [Google Scholar] [CrossRef]
- Sobral, F.; Calhelha, R.C.; Barros, L.; Dueñas, M.; Tomás, A.; Santos-Buelga, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Flavonoid Composition and Antitumor Activity of Bee Bread Collected in Northeast Portugal. Molecules 2017, 22, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dranca, F.; Ursachi, F.; Oroian, M. Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods 2020, 9, 1358. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Żukowska, R.; Naliwajko, S.K.; Bartosiuk, E.; Moskwa, J.; Isidorov, V.; Soroczyńska, J.; Borawska, M.H. Chemical composition and antioxidant activity of beebread, and its influence on the glioblastoma cell line (U87MG). J. Apic. Sci. 2013, 57, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.; Isidorova, A.; Sczczepaniak, L.; Czyżewska, U. Gas chromatographic–mass spectrometric investigation of the chemical composition of beebread. Food Chem. 2009, 115, 1056–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Jamali, M.A.; Peng, Z. Antioxidant Enzyme Activities and Lipid Oxidation in Rape (Brassica campestris L.) Bee Pollen Added to Salami during Processing. Molecules 2016, 21, 1439. [Google Scholar] [CrossRef]
- Chidinma, I.C.; Chigozie, I.J. Anti-Diabetic Effect of a Flavonoid and Sitosterol - Rich Aqueous Extract ofPleurotus tuberregiumSclerotia in Alloxan-Induced Diabetic Rabbits. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1148–1156. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.J.; Islam, N.; Jin, S.E.; Min, B.-S.; Lee, J.-H.; Sohn, H.S.; Choi, J.S. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch. Pharmacal Res. 2012, 35, 1021–1035. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and ?-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Pang, Y.-L.; Hu, J.-W.; Liu, G.-L.; Lu, S.-Y. Comparative medical characteristics of ZDF-T2DM rats during the course of development to late stage disease. Anim. Model. Exp. Med. 2018, 1, 203–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, H.; Man, F.; Guo, Z.; Xu, J.; Yan, W.; Li, J.; Pan, Q.; Wang, W. Sitagliptin Protects Cardiac Function by Reducing Nitroxidative Stress and Promoting Autophagy in Zucker Diabetic Fatty (ZDF) Rats. Cardiovasc. Drugs Ther. 2018, 32, 541–552. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Nwobodo, N.N.; Akpan, J.L.; Okorie, U.A.; Ezeonu, C.T.; Ezeokpo, B.C.; Nwadike, K.I.; Erhiano, E.; Wahab, M.S.A.; Sulaiman, S.A. Nigerian Honey Ameliorates Hyperglycemia and Dyslipidemia in Alloxan-Induced Diabetic Rats. Nutrients 2016, 8, 95. [Google Scholar] [CrossRef] [Green Version]
- Zaid, S.S.M.; Sulaiman, S.A.; Othman, N.H.; Soelaiman, I.-N.; Shuid, A.N.; Mohamad, N.; Muhamad, N. Protective effects of Tualang honey on bone structure in experimental postmenopausal rats. Clinics 2012, 67, 779–784. [Google Scholar] [CrossRef]
- Martiniaková, M.; Bobonova, I.; Omelka, R.; Duranova, H.; Babosová, R.; Stawarz, R.; Toman, R. Low administration of bee pollen in the diet affects qualitative histological characteristics of bone in male rats. Potravinarstvo Slovak J. Food Sci. 2014, 8, 277–283. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Knaga, S.; Dobrowolski, P.; Lamorski, K.; Jabłoński, M.; Tomczyk-Warunek, A.; Kadhim, M.J.; Hułas-Stasiak, M.; Borsuk, G.; Muszyński, S. The effect of bee pollen on bone biomechanical strength and trabecular bone histomorphometry in tibia of young Japanese quail (Coturnix japonica). PLoS ONE 2020, 15, e0230240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, M.; Hamamoto, R.; Uchiyama, S.; Ishiyama, K.; Hashimoto, K. Anabolic Effects of Bee Pollen Cistus ladaniferus Extract on Bone Components in the Femoral-Diaphyseal and -Metaphyseal Tissues of Rats in Vitro and in Vivo. J. Health Sci. 2006, 52, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Yudaniayanti, I.S.; Primarizky, H.; Nangoi, L.; Yuliani, G.A. Protective effects of honey by bees (Apis dorsata) on decreased cortical thickness and bone impact strength of ovariohysterectomized rats as models for menopause. Veter World 2019, 12, 868–876. [Google Scholar] [CrossRef]
- Jepsen, K.J.; Andarawis-Puri, N. The amount of periosteal apposition required to maintain bone strength during aging depends on adult bone morphology and tissue-modulus degradation rate. J. Bone Miner. Res. 2012, 27, 1916–1926. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.L.; Loveridge, N.; Reeve, J.; Thomas, C.D.; Feik, S.A.; Clement, J.G. Super-osteons (remodeling clusters) in the cortex of the femoral shaft: Influence of age and gender. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2001, 264, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Hui, K.; Hao, C.; Peng, Z.; Gao, Q.X.; Jin, Q.; Lei, G.; Min, J.; Qi, Z.; Bo, C.; et al. Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice. Connect. Tissue Res. 2016, 57, 277–289. [Google Scholar] [CrossRef]
- Stabley, J.N.; Prisby, R.D.; Behnke, B.J.; Delp, M.D. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J. Endocrinol. 2015, 225, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Qu, H.; Peng, J.; Luo, T.-Y.; Lv, F.-J.; Chen, L.; Wang, Z.-N.; Ouyang, Y.; Cheng, Q.-F. Abnormal spontaneous brain activity in type 2 diabetes with and without microangiopathy revealed by regional homogeneity. Eur. J. Radiol. 2016, 85, 607–615. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omelka, R.; Martiniakova, M.; Svik, K.; Slovak, L.; Payer, J.; Oppenbergerova, I.; Kovacova, V.; Babikova, M.; Soltesova-Prnova, M. The effects of eggshell calcium (Biomin H®) and its combinations with alfacalcidol (1α-hydroxyvitamin D3) and menaquinone-7 (vitamin K2) on ovariectomy-induced bone loss in a rat model of osteoporosis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Martiniaková, M.; Boboňová, I.; Omelka, R.; Grosskopf, B.; Stawarz, R.; Toman, R. Structural changes in femoral bone tissue of rats after subchronic peroral exposure to selenium. Acta Veter Scand. 2013, 55, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ricqles, A.J.; Meunier, F.J.; Castanet, J.; Francillon-Vieillot, H. Comparative microstructure of bone. In Bone Matrix and Bone Specific Products; CRC Press, Inc.: Boca Raton, FL, USA, 1991; Volume 3, pp. 1–78. [Google Scholar]
- Enlow, D.H.; Brown, S.O. A Comparative Histological Study of Fossil and Recent Bone Tissues. Part I. Tex. J. Sci. 1956, 8, 405–412. [Google Scholar]





| Group (Units) | Item | Content | Method |
|---|---|---|---|
| Main components (g/100 g) | Dry matter | 71.35 | GA |
| Protein | 19.27 | KM | |
| Fat | 6.48 | GA | |
| Carbohydrates | 2.09 | CAL | |
| Fibre | 41.25 | EGM | |
| Macroelements (g/kg) | Ca | 1.50 | ICP-AES |
| Mg | 0.91 | ICP-AES | |
| P | 4.48 | ICP-AES | |
| K | 4.55 | ICP-AES | |
| Na | 0.06 | ICP-AES | |
| Microelements (mg/kg) | Cu | 5.90 | F-AAS |
| Se | 0.21 | HG-AAS | |
| Cr | 0.14 | ETA-AAS | |
| Ni | 0.77 | ETA-AAS | |
| Co | <0.10 | ETA-AAS | |
| Vitamins (mg/kg) | A (retinol acetate) | <0.05 | HPLC-DAD |
| E (α-tocopherol acetate) | 28.4 | HPLC-DAD | |
| C (ascorbic acid) | <1.0 | HPLC-DAD | |
| B2 (riboflavin) | 9.6 | HPLC-DAD | |
| B3 (PP; nicotinamide) | 11.5 | HPLC-DAD | |
| Beta carotene | 16.5 | UV-VIS | |
| Fatty acids (g/100 g) | Saturated fatty acids | 3.91 | GC-FID |
| Monounsaturated fatty acids | 0.76 | GC-FID | |
| Polyunsaturated fatty acids | 0.81 | GC-FID | |
| Amino acids (g/kg) | Asp | 19.0 | IEC |
| Thr | 7.7 | IEC | |
| Ser | 7.1 | IEC | |
| Glu | 21.7 | IEC | |
| Pro | 15.0 | IEC | |
| Gly | 9.6 | IEC | |
| Ala | 9.6 | IEC | |
| Val | 10.5 | IEC | |
| Ile | 8.9 | IEC | |
| Leu | 13.7 | IEC | |
| Lys | 11.3 | IEC | |
| Arg | 7.8 | IEC | |
| Tyr | 4.3 | IEC | |
| Phe | 10.5 | IEC | |
| His | 5.2 | IEC | |
| Cys | 8.2 | IEC | |
| Met | 11.9 | IEC | |
| Trp | 2.2 | IEC |
| Type of Analysis | Parameter | Unit | Method |
|---|---|---|---|
| Biochemistry | blood glucose (BG) | mmol/L | POC glucometer 1 |
| blood insulin (BI) | μg/L | ELISA 2 | |
| total cholesterol (TC) | mmol/L | colorimetric 3 | |
| LDL cholesterol (LDLC) | mmol/L | colorimetric 3 | |
| HDL cholesterol (HDLC) | mmol/L | colorimetric 3 | |
| triglycerides (TG) | mmol/L | colorimetric 3 | |
| Cortical bone—3D imaging | relative bone volume (BV/TV) | % | micro-CT |
| bone mineral density (BMD) | mg HA/ccm | micro-CT | |
| cortical bone thickness (Ct.Th.) | mm | micro-CT | |
| bone surface (BS) | mm2 | micro-CT | |
| Cortical bone—2D imaging | area of primary osteons’ vascular canals (POVC) | μm2 | histomorphometry |
| area of Haversian canals (HC) | μm2 | histomorphometry | |
| area of secondary osteons (SO) | μm2 | histomorphometry | |
| Trabecular bone—3D imaging | relative bone volume (BV/TV) | % | micro-CT |
| trabecular number (Tb.N.) | 1/mm | micro-CT | |
| trabecular thickness (Tb.Th.) | mm | micro-CT | |
| bone mineral density (BMD) | mg HA/ccm | micro-CT | |
| bone surface (BS) | mm2 | micro-CT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martiniakova, M.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Omelka, R. Bee Bread Can Alleviate Lipid Abnormalities and Impaired Bone Morphology in Obese Zucker Diabetic Rats. Molecules 2021, 26, 2616. https://doi.org/10.3390/molecules26092616
Martiniakova M, Blahova J, Kovacova V, Babikova M, Mondockova V, Kalafova A, Capcarova M, Omelka R. Bee Bread Can Alleviate Lipid Abnormalities and Impaired Bone Morphology in Obese Zucker Diabetic Rats. Molecules. 2021; 26(9):2616. https://doi.org/10.3390/molecules26092616
Chicago/Turabian StyleMartiniakova, Monika, Jana Blahova, Veronika Kovacova, Martina Babikova, Vladimira Mondockova, Anna Kalafova, Marcela Capcarova, and Radoslav Omelka. 2021. "Bee Bread Can Alleviate Lipid Abnormalities and Impaired Bone Morphology in Obese Zucker Diabetic Rats" Molecules 26, no. 9: 2616. https://doi.org/10.3390/molecules26092616