Lipidic Matrixes Containing Clove Essential Oil: Biological Activity, Microstructural and Textural Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
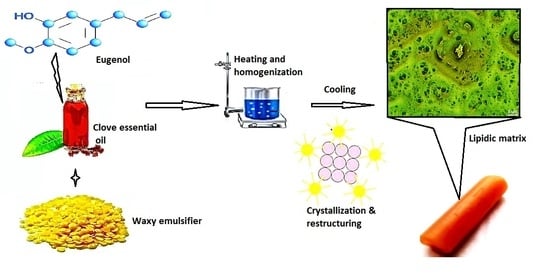
2.2. Preparation of Lipidic Matrixes
2.3. FT-IR Characterization
2.4. X-ray Diffraction
2.5. Surface Properties and Wetting Behavior
2.6. Thermal Behavior
2.7. Phase Contrast Microscopy
2.8. Total Phenolic Content
2.9. Antioxidant Activity by 1,1-Diphenyl-2-picrylhydrazyl Method (DDPH)
2.10. Antioxidant Activity by the Oxygen Radical Absorbance Capacity (ORAC)
2.11. Peroxide Value
2.12. Antimicrobial Activity
2.13. Mechanical Properties
2.14. Statistical Analysis
3. Results and Discussion
3.1. Crystallization Behavior
3.2. FT-IR Characterization
3.3. Crystal Morphology
3.4. Antioxidant Properties
3.5. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khalil, A.A.; Rahman, U.U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential Oil Eugenol: Sources, Extraction Techniques and Nutraceutical Perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Djouahria, A.; Boudareneab, L.; Meklatib, B.Y. Effect of extraction method on chemical composition, antioxidant and anti-inflammatory activities of essential oil from the leaves of algerian tetraclinis articulate. Ind. Crops Prod. 2013, 44, 32–36. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical composition and antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Fernandes de Souza, C.R.; Pereira-Oliveira, W. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, eugenia caryophyllata (Syzigium aromaticum L. myrtaceae): A short review. Phytotherapy Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- da Silva-Campelo, M.; Oliveira-Melo, E.; Pereira-Arrais, S.; Aires do Nascimento, F.B.S.; Viana-Gramos, N.; de Aguiar-Soares, S.; Pinho-Ribeiro, M.E.N.; Rocha-daSilva, C.; Nobre-Júnior, H.V. Clove essential oil encapsulated on nanocarrier based on polysaccharide: A strategy for the treatment of vaginal candidiasis. Colloid. Surf. Physicochem. Eng. Asp. 2021, 610, 1–11. [Google Scholar]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar]
- Duong, V.A.; Nguyen, T.T.L.; Maeng, H.J. Preparation of solid lipid nanoparticles and nanostructured lipid carriers for drug delivery and the effects of preparation parameters of solvent injection method. Molecules 2020, 18, 4781. [Google Scholar] [CrossRef]
- Chuberre, B.; Araviiskaia, E.; Bieber, T.; Barbaud, A. Mineral oils and waxes in cosmetics: An overview mainly based on the current european regulations and the safety profile of these compounds. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Freitas, C.; Müller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Petry, T.; Bury, D.; Fautz, R.; Hauser, M.; Huber, B.; Markowetz, A.; Mishra, S.; Rettinger, K.; Schuh, W.; Teichert, T. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicol. Lett. 2017, 280, 70–78. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Nesseem, D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. Int. J. Cosmet. Sci. 2011, 33, 70–79. [Google Scholar] [CrossRef]
- Galvão, J.G.; Santos, R.L.; Lira, A.A.M.; Kaminski, R.; Sarmento, V.H.; Severino, P.; Dolabella, S.S.; Scher, R.; Souto, E.B.; Nunes, R.S. Stearic acid, beeswax and carnauba wax as green raw materials for the loading of carvacrol into nanostructured lipid carriers. Appl. Sci. 2020, 10, 6267. [Google Scholar] [CrossRef]
- Shen, Q.; Li, W.; Li, W. The effect of clove oil on the transdermal delivery of ibuprofen in the rabbit by in vitro and in vivo methods. Drug Dev. Ind. Pharm. 2007, 33, 1369–1374. [Google Scholar] [CrossRef]
- El Mazouz, S.; Echchaoui, A.; Badrane, N.; Askour, M. Burning skin on face following the use of cloves (Syzygium aromaticum). Clin. Surg. 2017, 2, 1–2. [Google Scholar]
- Martinsa, A.J.; Cerqueira, M.A.; Fasolinc, L.H.; Cunhac, R.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Intern. 2016, 84, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Jana, S. Crystallization Behavior of Waxes. Ph.D. Thesis, Utah State University, Logan, UT, USA, 2016; p. 199. [Google Scholar]
- Berenice, O. Pectin-candelilla Wax: An Alternative Mixture for Edible films. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 167–171. [Google Scholar]
- Toro-Vasquez, J. Thermal and textural properties of organogels developed by candelilla wax in safflower oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Gomes-da Silva, J.Y.; Benjamin, S.R.; Florindo-Guedes, M.I.; Silva de Freitas, A.; Machado de Sousa, P.H.; Soares, D.J. Carnauba Wax uses in Food—A review. Food Chem. 2019, 291, 38–48. [Google Scholar]
- Zhang, Y.; Adams, M.; Zhang, Z.; Vidoni, O.; Leuenberger, B.H.; Achkar, J. Plasticisation of Carnauba Wax with Generally Recognized as Safe (GRAS) additives. Polymer 2016, 86, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Liebert, M.A. Final report on the safety assessment of fossil and synthetic waxes. J. Am. Coll. Toxicol. 1984, 3, 43–57. [Google Scholar]
- Greiveldinger, M.; Shanahan, M.E.R. Critique of the mathematical coherence of acid/base interfacial free energy theory. J. Colloid Interface 1999, 215, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Kupina, S.; Fields, C.; Roman, M.C.; Brunelle, S.L. Determination of total phenolic content using the folin-c assay: Single-laboratory validation. AOAC Intern. 2018, 101, 1466–1472. [Google Scholar] [CrossRef]
- Plank, D.W.; Szpylka, J.; Sapirstein, H.; Woollard, D.; Lee, V.; Oliver, C.Y.C.; Liu, R.H.; Tsao, R.; Düsterloh, A.; Baugh, S. Determination of antioxidant activity in foods and beverages by reaction with 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH): Collaborative Study. AOAC Intern. 2012, 95, 1562–1569. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, L.R. 1372. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe. J. AOAC Intern. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- AOAC Official Method 965.33. Peroxide value of oils and fats titration method. AOAC Off. Methods Anal. 2000, 12, 1. [Google Scholar]
- Cockerill, F.R.; Wikler, M.A. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In M07, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Clinical & Laboratory Standard Institute: Wayne, PA, USA, 2012; Volume 1, pp. 16–19. [Google Scholar]
- Marmur, A. Equilibrium contact angles: Theory and measurements. Colloids Surf. Physicochem. Eng. Aspects 1996, 116, 55–61. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the owen-wendt method. Int. J. Adhes 2009, 204, 451–457. [Google Scholar] [CrossRef]
- Li, S.; Zhai, S.; Lui, Y.; Zhou, H.; Wu, J.; Jiao, Q.; Zhang, B.; Zhu, H.; Yan, B. experimental modulation and computational model of nanohydrophobicity. Biomaterials 2015, 52, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Tavernier, I.; Doan, C.D.; de Walle, D.V.; Danthine, S.; Rimaux, T.; Dewettinck, K. Sequential crystallization of high and low melting waxes to improve oil structuring in wax based oleogels. RSC Adv. 2017, 7, 12113–12125. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, S.; Rojas, J. Essential oils structuring: A developing strategy to preserve their biological activity and improve their stability. J. Pharm. Sci. Res. 2020, 12, 1364–1370. [Google Scholar]
- Rodríguez, J.D.W.; Peyron, S.; Rigou, P.; Chalier, P. Rapid quantification of clove (Syzygium aromaticum) and spearmint (Mentha spicata) essential oils encapsulated in a complex organic matrix using an ATR-FTIR spectroscopic method. PLoS ONE 2018, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Taraj, K.; Andoni, A.; Ylli, F.; Malollari, I. Spectroscopic investigation of Syzygium aromaticum L. oil by water distillation extraction. J. Intern. Environ. Appl. Sci. 2019, 15, 122–126. [Google Scholar]
- Shende, P.K.; Gaud, R.S.; Bakal, R.; Yeole, Y. Clove oil emulsified buccal patch of serratiopeptidase for controlled release in toothache. J. Bioequi. Availab. 2016, 8, 134–139. [Google Scholar]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of beeswax, candelilla wax and paraffin wax for coating cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Vyshniak, V.; Dimitriev, O.; Litvynchuk, S.; Dombrovskiy, V. Identification of beeswax and its falsification by the method of infrared spectroscopy food technology. Ukr. Food J. 2018, 7, 421–433. [Google Scholar] [CrossRef]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Gajger, I.T. An approach for routine analytical detection of beeswax adulteration using FTIR-ATR spectroscopy. J. Apic. Sci. 2015, 59, 1–14. [Google Scholar] [CrossRef] [Green Version]
- de Castro, P.; Silva, L.A.; Ribeiro, G.; Marconcini, J.M. Production and stability of carnauba wax nanoemulsion. Adv. Sci. Eng. Med. 2017, 9, 977–985. [Google Scholar]
- Rojas, J.; Cabrera, S.; Ciro, G.; Naranjo, A. Lipidic matrixes containing lemon essential oil increases storage stability: Reological, thermal, and microstructural studies. Appl. Sci. 2020, 10, 3909. [Google Scholar] [CrossRef]
- Bentaye, K.; Vera, P.; Rubio, C.; Nerín, C. The additive properties of oxygen radical absorbance capacity (ORAC) assay: The case of essential oils food chemistry. Food Chem. 2014, 148, 204–208. [Google Scholar]
- Yoshimura, M.; Amakura, Y.; Yoshida, T. Polyphenolic compounds in clove and pimento and their antioxidative activities. Biosci. Biotechnol. Biochem. 2011, 75, 2207–2212. [Google Scholar] [CrossRef] [Green Version]
- Ghadermazi, R.; Sayed, H.G.; Goli, A.H. Antioxidant activity of clove (Eugenia caryophyllata Thunb), oregano (Oringanum vulgare L.) and sage (Salvia officinalis L.) essential oils in various model systems. Intern. Food Res. J. 2016, 24, 1628–1635. [Google Scholar]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, quality, and antioxidant activity of clove (Syzygium aromaticum L.) bud oil at the different phenological stages in young and mature trees. Hindawi Scien. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Muhson, I.; Mashkor, A.L. Evaluation of antioxidant activity of clove (Syzygium aromaticum). Int. J. Chem. Sci. 2015, 13, 23–30. [Google Scholar]
- Gülçin, I. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food. 2010, 14, 975–985. [Google Scholar] [CrossRef]
- D′ Avila-Farias, M.; Souto-Oliveira, P.; Pereira-Dutra, F.; Jacobsen-Fernandes, T.; de Pereira, C.; Quintana-de Oliveira, S.; Moro-Stefanello, F.; Leonetti-Lencina, C.; Gatto-Barschak, A. Eugenol derivatives as potential anti-oxidants: Is phenolic hydroxyl necessary to obtain an effect? J. Pharm. Pharmacol. 2014, 66, 733–746. [Google Scholar] [CrossRef]
- Mehmet, M.Ö.; Arslan, D. Antioxidant effect of essential oils of rosemary, clove and cinnamon on hazelnut and poppy oils. Food Chem. 2011, 129, 171–174. [Google Scholar]
- Fatemeh, E.; Khadijeh, K.; Aziz, Z.; Fatemeh, M.N.; Yeganeh, S.; Zahra, S.; Hedayat. H.; Manochehr, B. Evaluating the rancidity and quality of discarded oils in fast food restaurants. Food Sci. Nut. 2019, 7, 2302–2311. [Google Scholar]
- Setyaningsih, D.; Siahaan, D.G. The influence of essential oil addition to oxidative stability of palm oil biodisel. Conf. Ser. Earth Environ. Sci. 2007, 209, 1–9. [Google Scholar]
- Nuñez, L.; Aquino, M.D. Microbicide activity of clove essential oil (Eugenia Caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res. 2007, 21, 989–994. [Google Scholar] [CrossRef]






| Waxy Emulsifier | Aliphatic Ester (%) | Hydroxy Esters (%) | Diesters (%) | Hydrocarbon (%) | Fatty Alcohol (%) | Free Fatty Acids (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Beeswax | C40–0: 35 | C24–34: 12 | C54–68: 17 | C21–35: 14 | C24–36: 1 | C16,C24,C34: 14 | [20,21] |
| Candelilla | Simple esters-lactones: 21 | 6–8 | 0 | hentriacontane: 46–46.5 | 12–14 | 5 | [22,23] |
| nonacosane: 2.5 | |||||||
| triacontane: 2.5 | |||||||
| Carnauba | C50: 38–40 | 13 | * 4-HCA: 21 ** 4-MCA: 7 | C27–28: 1 | C32–34: 12 | C16: 5 | [24,25] |
| Ozokerite | 0 | 0 | 0 | asphaltens: 1–3 | 0 | 0 | [26] |
| cerecin: 40–60 | |||||||
| mineral oil: 25–45 | |||||||
| parafin: 1 | |||||||
| petroleum resins: 12 |
| Wax Material or Lipidic Matrix | DC (%) | (110°) | d-Spacing (nm) | (200°) | d-Spacing (nm) | Total SFE (mN/m) | SFEd (mN/m) | SFEp (mN/m) | r2 | Ip/d | Wadh (mN/m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BEW | 53.7 | 21.35 | 0.417 | 23.71 | 0.376 | * 20.4 ± 1.0 | 10.0 ± 0.0 | 10.4 ± 1.0 | 1.00 | 1.04 | 74.6 ± 1.5 |
| OKW | 67.7 | 21.35 | 0.417 | 23.71 | 0.376 | * 20.5 ± 1.1 | * 20.4 ± 1.1 | * 0.05 ± 0.0 | 0.91 | 2.45 | * 45.5 ± 5.1 |
| CRW | 70.6 | 21.40 | 0.416 | 23.71 | 0.376 | 116.5 ± 6.9 | 116.5 ± 6.9 | * 0.0 ± 0.0 | 0.89 | * 0.00 | * 47.2 ± 15.3 |
| CLW | 67.1 | 21.4 | 0.416 | 23.77 | 0.375 | * 18.8 ± 1.5 | * 18.0± 1.4 | * 0.8 ± 0.0 | 0.94 | * 0.04 | * 51.0 ± 1.2 |
| BEW matrix | 29.0 | 21.47 | 0.415 | 23.88 | 0.374 | 47.0 ± 4.1 | 5.7 ± 0.1 | 41.3 ± 4.0 | 0.96 | 7.24 | 113.3 ±5.5 |
| CRW matrix | 28.5 | 21.41 | 0.416 | 23.78 | 0.375 | * 26.1 ± 1.2 | 3.5 ± 0.1 | 22.6 ± 1.1 | 0.95 | 6.45 | 85.1 ± 2.5 |
| CLW matrix | 20.6 | 21.52 | 0.414 | 23.88 | 0.374 | * 23.9 ± 1.2 | * 17.0 ± 1.0 | 6.9 ± 0.1 | 0.99 | * 0.41 | 74.5 ± 8.7 |
| OKWmatrix | 31.6 | 21.47 | 0.415 | 23.83 | 0.374 | 31.4 ± 2.2 | * 14.9 ± 1.1 | 16.4 ± 0.1 | 0.91 | 1.10 | 92.3 ± 2.8 |
| p-value | N.A. | N.A. | N.A. | N.A. | N.A. | 0.00 | 0.00 | 0.00 | N.A. | 0.00 | 0.00 |
| Wax Material or Lipidic Matrix | Tm Onset (°C) | Tm (°C) | Tm offset (°C) | Tc onset (°C) | Tc (°C) | Tc offset (°C) | ∆T (°C) | ΔHm (J/g) | ΔHc (J/g) |
|---|---|---|---|---|---|---|---|---|---|
| BEW | 31.4 | 37.8 | 43.8 | 57.7 | 53.9 | 48.5 | 13.9 | 26.0 | 0.8 |
| CLW | 25.7 | 29.3 | 33.2 | 57.0 | 59.9 * | 45.4 | 23.8 | 5.6 | 1.3 |
| 55.5 | 67.4 | 72.3 | 65.7 | 61.0 | 57.0 | 6.6 | 6.6 | 1.6 | |
| CRW | 60.0 | 82 | 85.0 | 71.7 | 61.5 | 51.7 | 13.3 | 17.0 | 0.4 |
| OKW | 70.1 | 76.6 | 79.0 | 65.8 | 64.0 | 57.9 | 13.2 | 29.2 | 1.1 |
| BEW matrix | 4.0 51.9 77.0 | 7.1 57.9 74.6 | 8.4 60.7 82.8 | - 61.5 79.5 | - 50.7 74.6 | - 35.2 70.9 | - 0.8 2.5 | 0.9 3.0 0.34 | - 2.8 0.9 |
| CLW matrix | 2.9 22.6 53.5 | 9.0 27.5 58.7 | 11.0 29.2 62.1 | - 56.8 80.9 | - 52.3 75.8 | - 40.3 71.7 | - 27.6 18.8 | 1.2 3.5 3.8 | - 4.4 2.2 |
| CRW matrix | 3.1 40.4 50.3 65.5 | 7.2 47.5 51.8 74.6 | 12.3 50.7 57.9 79.1 | - 52.3 64.0 81.6 | - 45.0 58.6 77.5 | - 38.4 55.3 76.1 | - 1.6 6.1 2.5 | 0.5 2.4 5.0 7.3 | - 1.6 0.4 1.7 |
| OKW matrix | 3.2 35.8 42.0 61.0 | 5.9 41.1 47.4 63.9 | 13.6 42.0 50.2 66.9 | - - 67.1 81.2 | - - 58.5 75.6 | - - 55.3 71.8 | - - 16.9 14.3 | 0.3 3.3 5.8 1.6 | - - 2.1 2.3 |
| Sample | Total Phenolic Content (mg Gallic Acid/100 g) | DDPH (mg Trolox/100 g) | ORAC (mg Trolox/100 g) | Peroxide Value (meq Oxygen/kg) |
|---|---|---|---|---|
| BEW matrix | 480,165.6 ± 18.3 | 8504.1 ± 66.5 | 247,129.8 ± 0.9 | 0 ± 0 |
| CRW matrix | 427,453.2 ± 18.2 | 7209.9 ± 45.7 | 205,140.7 ± 0.8 | 0 ± 0 |
| CLW matrix | 383,993.4 ± 14.8 | 6765.1 ± 47.3 | 170,429.0 ± 0.0 | 0 ± 0 |
| OKW matrix | 370,787.4 ± 15.0 | 5143.8 ± 55.3 | 159,944.7 ± 1.0 | 0 ± 0 |
| CEO | 7289.9 ± 11.3 | 12,002.2 ± 44.3 | N.R.* | 13.02 ± 0.01 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 |
| Wax Material or Lipidic Matrix | Mechanical Properties | ||||
|---|---|---|---|---|---|
| Elastic Energy (J) | Stiffness (N/mm) | Ultimate Strength (N) | Elastic Limit (mm) | Toughness (J) | |
| Beewax | 22.3 | 57.6 | 48.7 | 0.9 | 25.4 |
| Candelilla | 6.1 | 196.0 | 50.3 | 0.3 | 8.5 |
| Carnauba | 6.0 | 203 | 50.4 | 0.32 | 9.0 |
| Ozokerite | 17.7 | 70.5 | 49.0 | 0.72 | 21.7 |
| BEW matrix | 0.11 | 0.40 | 0.30 | 0.62 | 0.12 |
| CLW matrix | 0.006 | 3.3 | 0.39 | 0.04 | 0.011 |
| CRW matrix | 0.022 | 0.04 | 0.06 | 0.74 | 0.025 |
| OKW matrix | 0.04 | 0.98 | 0.28 | 0.26 | 0.05 |
| Commercial product | 0.01 | 6.0 | 0.37 | 0.1 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, J.; Cabrera, S.; Benavides, J.; Lopera, Y.; Yarce, C.J. Lipidic Matrixes Containing Clove Essential Oil: Biological Activity, Microstructural and Textural Studies. Molecules 2021, 26, 2425. https://doi.org/10.3390/molecules26092425
Rojas J, Cabrera S, Benavides J, Lopera Y, Yarce CJ. Lipidic Matrixes Containing Clove Essential Oil: Biological Activity, Microstructural and Textural Studies. Molecules. 2021; 26(9):2425. https://doi.org/10.3390/molecules26092425
Chicago/Turabian StyleRojas, John, Sergio Cabrera, Julie Benavides, Yasmín Lopera, and Cristhian J. Yarce. 2021. "Lipidic Matrixes Containing Clove Essential Oil: Biological Activity, Microstructural and Textural Studies" Molecules 26, no. 9: 2425. https://doi.org/10.3390/molecules26092425