Precise Synthesis and Thin Film Self-Assembly of PLLA-b-PS Bottlebrush Block Copolymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Macromonomers
2.2. Sequential ROMP of Macromonomers
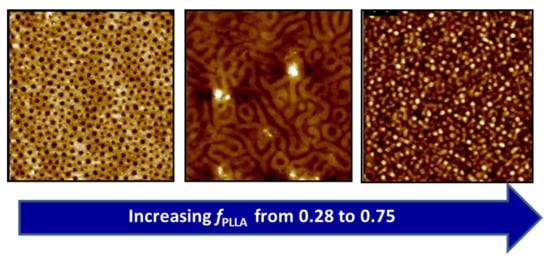
2.3. Thin Film Self-Assembly of BBCPs
2.4. Solvent Effects on PLLA-b-PS BBCP Thin Film Self-Assembly
2.5. Generation of Nanoporous Thin-Films
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xia, Y.; Olsen, B.D.; Kornfield, J.A.; Grubbs, R.H. Efficient synthesis of narrowly dispersed brush copolymers and study of their assemblies: The importance of side chain arrangement. J. Am. Chem. Soc. 2009, 131, 18525–18532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, G.M.; Weitekamp, R.A.; Piunova, V.A.; Grubbs, R.H. Synthesis of isocyanate-based brush block copolymers and their rapid self-assembly to infrared-reflecting photonic crystals. J. Am. Chem. Soc. 2012, 134, 14249–14254. [Google Scholar] [CrossRef]
- Li, X.; Prukop, S.L.; Biswal, S.L.; Verduzco, R. Surface properties of bottlebrush polymer thin films. Macromolecules 2012, 45, 7118–7127. [Google Scholar] [CrossRef]
- Sveinbjörnsson, B.R.; Weitekamp, R.A.; Miyake, G.M.; Xia, Y.; Atwater, H.A.; Grubbs, R.H. Rapid self-assembly of brush block copolymers to photonic crystals. Proc. Natl. Acad. Sci. USA 2012, 109, 14332–14336. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Cho, S.; Clark, C.; Verkhoturov, S.V.; Eller, M.J.; Li, A.; Pavía-Jiménez, A.; Schweikert, E.A.; Thackeray, J.W.; Trefonas, P.; et al. Nanoscopic cylindrical dual concentric and lengthwise block brush terpolymers as covalent preassembled high-resolution and high-sensitivity negative-tone photoresist materials. J. Am. Chem. Soc. 2013, 135, 4203–4206. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman-Martin, A.L.; Chu, C.K.; Grubbs, R.H. Application of Bottlebrush Block Copolymers as Photonic Crystals. Macromol. Rapid Commun. 2017, 38, 1700058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.V.T.; Detappe, A.; Harvey, P.; Gallagher, N.; Mathieu, C.; Agius, M.P.; Zavidij, O.; Wang, W.; Jiang, Y.; Rajca, A.; et al. Pro-organic radical contrast agents (“pro-ORCAs”) for real-time MRI of pro-drug activation in biological systems. Polym. Chem. 2020, 11, 4768–4779. [Google Scholar] [CrossRef]
- Vohidov, F.; Milling, L.E.; Chen, Q.; Zhang, W.; Bhagchandani, S.; Nguyen, H.V.-T.; Irvine, D.J.; Johnson, J.A. ABC triblock bottlebrush copolymer-based injectable hydrogels: Design, synthesis, and application to expanding the therapeutic index of cancer immunochemotherapy. Chem. Sci. 2020, 11, 5974–5986. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Rzayev, J. Synthesis of polystyrene-polylactide bottlebrush block copolymers and their melt self-assembly into large domain nanostructures. Macromolecules 2009, 42, 2135–2141. [Google Scholar] [CrossRef]
- Nikovia, C.; Theodoridis, L.; Alexandris, S.; Bilalis, P.; Hadjichristidis, N.; Floudas, G.; Pitsikalis, M. Macromolecular Brushes by Combination of Ring-Opening and Ring-Opening Metathesis Polymerization. Synthesis, Self-Assembly, Thermodynamics, and Dynamics. Macromolecules 2018, 51, 8940–8955. [Google Scholar] [CrossRef]
- Rajaram, S.; Choi, T.L.; Rolandi, M.; Fréchet, J.M.J. Synthesis of dendronized diblock copolymers via ring-opening metathesis polymerization and their visualization using atomic force microscopy. J. Am. Chem. Soc. 2007, 129, 9619–9621. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Kornfield, J.A.; Grubbs, R.H. Efficient synthesis of narrowly dispersed brush polymers via living ring-opening metathesis polymerization of macromonomers. Macromolecules 2009, 42, 3761–3766. [Google Scholar] [CrossRef] [Green Version]
- Zhulina, E.B.; Sheiko, S.S.; Dobrynin, A.V.; Borisov, O.V. Microphase Segregation in the Melts of Bottlebrush Block Copolymers. Macromolecules 2020, 53, 2582–2593. [Google Scholar] [CrossRef]
- Runge, M.B.; Bowden, N.B. Synthesis of high molecular weight comb block copolymers and their assembly into ordered morphologies in the solid state. J. Am. Chem. Soc. 2007, 129, 10551–10560. [Google Scholar] [CrossRef] [PubMed]
- Brett Runge, M.; Lipscomb, C.E.; Ditzler, L.R.; Mahanthappa, M.K.; Tivanski, A.V.; Bowden, N.B. Investigation of the assembly of comb block copolymers in the solid state. Macromolecules 2008, 41, 7687–7694. [Google Scholar] [CrossRef]
- Gu, W.; Huh, J.; Hong, S.W.; Sveinbjornsson, B.R.; Park, C.; Grubbs, R.H.; Russell, T.P. Self-assembly of symmetric brush diblock copolymers. ACS Nano. 2013, 7, 2551–2558. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.W.; Gu, W.; Huh, J.; Sveinbjornsson, B.R.; Jeong, G.; Grubbs, R.H.; Russell, T.P. On the self-assembly of brush block copolymers in thin films. ACS Nano. 2013, 7, 9684–9692. [Google Scholar] [CrossRef] [Green Version]
- Dalsin, S.J.; Rions-Maehren, T.G.; Beam, M.D.; Bates, F.S.; Hillmyer, M.A.; Matsen, M.W. Bottlebrush Block Polymers: Quantitative Theory and Experiments. ACS Nano. 2015, 9, 12233–12245. [Google Scholar] [CrossRef]
- Kim, M.J.; Yu, Y.G.; Chae, C.G.; Seo, H.B.; Bak, I.G.; Mallela, Y.L.N.K.; Lee, J.S. ω-Norbornenyl Macromonomers: In Situ Synthesis by End-Capping of Living Anionic Polymers Using a Norbornenyl-Functionalized α-Phenyl Acrylate and Their Ring-Opening Metathesis Polymerization. Macromolecules 2019, 52, 103–112. [Google Scholar] [CrossRef]
- Jiang, L.; Nykypanchuk, D.; Ribbe, A.E.; Rzayev, J. One-Shot Synthesis and Melt Self-Assembly of Bottlebrush Copolymers with a Gradient Compositional Profile. ACS Macro. Lett. 2018, 619–623. [Google Scholar] [CrossRef]
- Gai, Y.; Song, D.P.; Yavitt, B.M.; Watkins, J.J. Polystyrene-block-poly(ethylene oxide) Bottlebrush Block Copolymer Morphology Transitions: Influence of Side Chain Length and Volume Fraction. Macromolecules 2017, 50, 1503–1511. [Google Scholar] [CrossRef]
- Cho, S.; Son, J.; Kim, I.; Ahn, H.; Jang, H.S.; Joo, S.H.; Park, K.H.; Lee, E.; Kim, Y.; Ahn, S.K. Asymmetric polystyrene-polylactide bottlebrush random copolymers: Synthesis, self-assembly and nanoporous structures. Polymer 2019, 175, 49–56. [Google Scholar] [CrossRef]
- Sunday, D.F.; Dolejsi, M.; Chang, A.B.; Richter, L.J.; Li, R.; Kline, R.J.; Nealey, P.F.; Grubbs, R.H. Confinement and Processing Can Alter the Morphology and Periodicity of Bottlebrush Block Copolymers in Thin Films. ACS Nano. 2020, 14, 17476–17486. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.M.; Chiang, Y.W.; Tsai, C.C.; Lin, C.C.; Ko, B.T.; Huang, B.H. Three-Dimensionally Packed Nanohelical Phase in Chiral Block Copolymers. J. Am. Chem. Soc. 2004, 126, 2704–2705. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.M.; Chen, C.K.; Chiang, Y.W.; Ko, B.T.; Lin, C.C. Tubular nanostructures from degradable core-shell cylinder microstructures in chiral diblock copolymers. Adv. Mater. 2006, 18, 2355–2358. [Google Scholar] [CrossRef]
- Ho, R.M.; Chen, C.K.; Chiang, Y.W. Novel nanostructures from self-assembly of chiral block copolymers. Macromol. Rapid. Commun. 2009, 30, 1439–1456. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.A.; Hillmyer, M.A. Nanoporous membranes derived from block copolymers: From drug delivery to water filtration. ACS Nano. 2010, 4, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Vayer, M.; Hillmyer, M.A.; Dirany, M.; Thevenin, G.; Erre, R.; Sinturel, C. Perpendicular orientation of cylindrical domains upon solvent annealing thin films of polystyrene-b-polylactide. Thin. Solid. Films. 2010, 518, 3710–3715. [Google Scholar] [CrossRef]
- Theodosopoulos, G.V.; Bitsi, S.L.; Pitsikalis, M. Complex Brush-Like Macromolecular Architectures via Anionic and Ring Opening Metathesis Polymerization: Synthesis, Characterization, and Thermal Properties. Macromol. Chem. Phys. 2018, 219, 1700253. [Google Scholar] [CrossRef]
- Rule, J.D.; Moore, J.S. ROMP reactivity of endo- and exo-dicyclopentadiene. Macromolecules 2002, 35, 7878–7882. [Google Scholar] [CrossRef]
- Grubisic, Z.; Rempp, P.; Benoit, H. A universal calibration for gel permeation chromatography. J. Polym. Sci. Part. B. Polym. Lett. 1967, 5, 753–759. [Google Scholar] [CrossRef]
- Olayo-Valles, R.; Guo, S.; Lund, M.S.; Leighton, C.; Hillmyer, M.A. Perpendicular Domain Orientation in Thin Films of Polystyrene−Polylactide Diblock Copolymers. Macromolecules 2005, 38, 10101–10108. [Google Scholar] [CrossRef]
- Baruth, A.; Seo, M.; Lin, C.H.; Walster, K.; Shankar, A.; Hillmyer, M.A.; Leighton, C. Optimization of long-range order in solvent vapor annealed poly(styrene)-block-poly(lactide) thin films for nanolithography. ACS Appl. Mater. Interfaces 2014, 6, 13770–13781. [Google Scholar] [CrossRef]
- Sinturel, C.; Grosso, D.; Boudot, M.; Amenitsch, H.; Hillmyer, M.A.; Pineau, A.; Vayer, M. Structural transitions in asymmetric poly(styrene)-block -poly(lactide) thin films induced by solvent vapor exposure. ACS Appl. Mater. Interfaces 2014, 6, 12146–12152. [Google Scholar] [CrossRef]
- Cummins, C.; Mokarian-Tabari, P.; Andreazza, P.; Sinturel, C.; Morris, M.A. Solvothermal Vapor Annealing of Lamellar Poly(styrene)-block-poly(d,l-lactide) Block Copolymer Thin Films for Directed Self-Assembly Application. ACS Appl. Mater. Interfaces 2016, 8, 8295–8304. [Google Scholar] [CrossRef]
- She, M.S.; Lo, T.Y.; Hsueh, H.Y.; Ho, R.M. Nanostructured thin films of degradable block copolymers and their applications. NPG Asia Mater. 2013, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Ho, R.M.; Tseng, W.H.; Fan, H.W.; Chiang, Y.W.; Lin, C.C.; Ko, B.T.; Huang, B.H. Solvent-induced microdomain orientation in polystyrene-b-poly(l-lactide) diblock copolymer thin films for nanopatterning. Polymer 2005, 46, 9362–9377. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Gowd, E.B.; Böhme, M.; Stamm, M. In Situ GISAXS Study on Solvent Vapour Induced Orientation Switching in PS- b -P4VP Block Copolymer Thin Films. IOP Conf. Ser. Mater. Sci. Eng. 2010, 14, 012015. [Google Scholar] [CrossRef]
- Cummins, C.; Mokarian-Tabari, P.; Holmes, J.D.; Morris, M.A. Selective etching of polylactic acid in poly(styrene)-block-poly(d,l) lactide diblock copolymer for nanoscale patterning. J. Appl. Polym. Sci. 2014, 131, 9493–9504. [Google Scholar] [CrossRef]
- Keen, I.; Yu, A.; Cheng, H.-H.; Jack, K.S.; Nicholson, T.M.; Whittaker, A.K.; Blakey, I. Control of the orientation of symmetric poly (styrene)-block-poly (D, L-lactide) block copolymers using statistical copolymers of dissimilar composition. Langmuir 2012, 28, 15876–15888. [Google Scholar] [CrossRef] [PubMed]
- Keen, I.; Cheng, H.-H.; Yu, A.; Jack, K.S.; Younkin, T.R.; Leeson, M.J.; Whittaker, A.K.; Blakey, I. Behavior of Lamellar Forming Block Copolymers under Nanoconfinement: Implications for Topography Directed Self-Assembly of Sub-10 nm Structures. Macromolecules 2014, 47, 276–283. [Google Scholar] [CrossRef]
- Stefik, M.; Guldin, S.; Vignolini, S.; Wiesner, U.; Steiner, U. Block copolymer self-assembly for nanophotonics. Chem. Soc. Rev. 2015, 44, 5076–5091. [Google Scholar] [CrossRef] [Green Version]
- Cummins, C.; Lundy, R.; Walsh, J.J.; Ponsinet, V.; Fleury, G.; Morris, M.A. Enabling future nanomanufacturing through block copolymer self-assembly: A review. Nano. Today 2020, 35, 100936. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y. Selective Swelling of Block Copolymers: An Upscalable Greener Process to Ultrafiltration Membranes? Macromolecules 2020, 53, 5–17. [Google Scholar] [CrossRef]





| Mn,SEC (Da) a | Ð a | Mn,NMR (Da) b | DPNMR b | Mn,MALDI (Da) c | Ð c | |
|---|---|---|---|---|---|---|
| NB-PLLA2.58 | 2580 | 1.08 | 1850 | 12 | - | - |
| NB-PLLA3.68k | 3680 | 1.16 | 2570 | 17 | 2660 | 1.05 |
| NB-PS3.28k | 3280 | 1.40 | 3450 | 31 | 3260 | 1.10 |
| MM1 a | MM2 a | [MM1/C]: [MM2/C] | Mn (kDa) b | Ð a | fPLLAc | Domain Size (nm) d | Period (nm) d | Morphology d | |
|---|---|---|---|---|---|---|---|---|---|
| P1 | PLLA3.68k | PS3.28k | 30:75 | 301 | 1.25 | 0.28 | 46 | 72 | PS C⊥ |
| P2 | PLLA3.68k | PS3.28k | 30:60 | 275 | 1.28 | 0.37 | 51 | 92 | PS C∥ |
| P3 | PLLA2.56k | PS3.28k | 30:30 | 171 | 1.20 | 0.47 | 58 | 102 | L⊥ |
| P4 | PLLA3.68k | PS3.28k | 30:15 | 220 | 1.14 | 0.75 | - | 70 | PLLA C⊥ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, E.; Cummins, C.; Fleury, G. Precise Synthesis and Thin Film Self-Assembly of PLLA-b-PS Bottlebrush Block Copolymers. Molecules 2021, 26, 1412. https://doi.org/10.3390/molecules26051412
Ji E, Cummins C, Fleury G. Precise Synthesis and Thin Film Self-Assembly of PLLA-b-PS Bottlebrush Block Copolymers. Molecules. 2021; 26(5):1412. https://doi.org/10.3390/molecules26051412
Chicago/Turabian StyleJi, Eunkyung, Cian Cummins, and Guillaume Fleury. 2021. "Precise Synthesis and Thin Film Self-Assembly of PLLA-b-PS Bottlebrush Block Copolymers" Molecules 26, no. 5: 1412. https://doi.org/10.3390/molecules26051412