Antimalarials and Phytotoxins from Botryosphaeria dothidea Identified from a Seed of Diseased Torreya taxifolia
Abstract
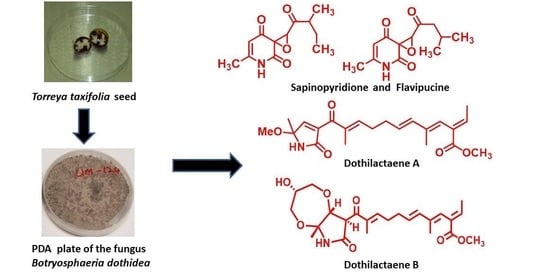
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Isolation of the Fungus from a Seed of Diseased T. taxifolia
3.3. Identification of the Fungus by DNA Analysis
3.4. Fermentation, Extraction, and Purification
3.5. In Vitro Antiplasmodial Assay
3.6. In Vitro Phytotoxicity Assay
3.7. In Vitro Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lim, L.; McFadden, G.I. The evolution, metabolism, and functions of the apicoplast. Phil. Trans. R. Soc. B 2010, 365, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arisue, N.; Hashimoto, T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015, 64, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.A.; van Dooren, G.G.; Waller, R.F.; Crawford, M.J.; Fraunholz, M.J.; Foth, B.J.; Tonkin, C.J.; Roos, D.S.; McFadden, G.I. Tropical infectious diseases: Metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Micro. 2004, 2, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ralph, S.A.; D’Ombrain, M.C.; McFadden, G.I. The apicoplast as an antimalarial drug target. Drug Resist. Update. 2001, 4, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botté, C.Y.; Dubar, F.; McFadden, G.I.; Maréchal, E.; Biot, C. Plasmodium falciparum apicoplast drugs: Targets or off-targets? Chem. Rev. 2012, 112, 1269–1283. [Google Scholar] [CrossRef]
- Uddin, T.; McFadden, G.I.; Goodman, C.D. Validation of putative apicoplast-targeting drugs using a chemical supplementation assay in cultured human malaria parasites. Antimicrob. Agents Chemother. 2018, 62, e01161-17. [Google Scholar] [CrossRef] [Green Version]
- Franck, E.D.; Duke, S. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar]
- Bajsa, J.; Singh, K.; Nanayakkara, D.; Duke, S.O.; Rimando, A.M.; Evidente, A.; Tekwani, B.L. A Survey of synthetic and natural phytotoxic compounds and phytoalexins as potential antimalarial compounds. Biol. Pharm. Bull. 2007, 30, 1740–1744. [Google Scholar] [CrossRef] [Green Version]
- Herath, H.M.B.T.; Herath, W.H.M.W.; Carvalho, P.; Khan, S.I.; Tekwani, B.L.; Duke, S.O.; Tomaso-Peterson, M.; Nanayakkara, N.P.D. Biologically active tetranorditerpenoids from the fungus Sclerotinia homoeocarpa causal agent of dollar spot in turfgrass. J. Nat. Prod. 2009, 72, 2091–2097. [Google Scholar] [CrossRef] [Green Version]
- Kumarihamy, M.; Fronczek, F.R.; Ferreira, D.; Jacob, M.; Khan, S.I.; Nanayakkara, N.P.D. Bioactive 1,4-dihydroxy-5-phenyl-2-pyridinone alkaloids from Septoria pistaciarum. J. Nat. Prod. 2010, 73, 1250–1253. [Google Scholar] [CrossRef] [Green Version]
- Kumarihamy, M.; Khan, S.I.; Jacob, M.; Tekwani, B.L.; Duke, S.O.; Ferreira, D.; Nanayakkara, N.P.D. Antiprotozoal and antimicrobial compounds from Septoria pistaciarum. J. Nat. Prod. 2012, 75, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumarihamy, M.; Ferreira, D.; Croom, E.M., Jr.; Sahu, R.; Tekwani, B.L.; Duke, S.O.; Khan, S.; Techen, N.; Nanayakkara, N.P.D. Antiplasmodial and cytotoxic cytochalasins from an endophytic fungus, Nemania sp. UM10M, isolated from a diseased Torreya taxifolia leaf. Molecules 2019, 24, 777. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.W.; Hermann, S.M. The continuing population decline of Torreya taxifolia Arn. Bull. Torrey Bot. Club 1993, 120, 275–328. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Hermann, S.M.; Vogel, C. The catastrophic loss of Torreya taxifolia: Assessing environmental induction of disease hypotheses. Ecol. Appl. 1995, 5, 501–516. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Hermann, S.M.; van Mantgem, P.J. Estimating the magnitude of decline of the Florida torreya (Torreya taxifolia Arn.). Biol. Conserve. 2000, 95, 77–84. [Google Scholar] [CrossRef]
- Lee, J.C.; Yang, X.; Schwartz, M.; Strobel, G.; Clardy, J. The relationship between an endangered North American tree and an endophytic fungus. Chem. Biol. 1995, 2, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.A.; O’Donnell, K.; Mount, L.L.; Shin, K.; Detemann, R. A novel Fusarium species causes a canker disease of the critically endangered conifer, Torreya taxifolia. Plant Dis. 2011, 95, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, T.; Smith, J.A.; Mount, L.L.; Geiser, D.M.; O’Donnell, K. Fusarium torreyae sp. nov., a pathogen causing canker disease of Florida torreya (Torreya taxifolia), a critically endangered conifer restricted to northern Florida and southwestern Georgia. Mycologia 2013, 105, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Groenewald, J.Z.; Cheewangkoon, R.; Jami, F.; Abdollahzadeh, J.; Lombard, L.; Crous, P.W. Families, genera, and species of Botryosphaeriales. Fungal Biol. 2017, 121, 322–346. [Google Scholar] [CrossRef]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Onuma, Y. Isolation and identification of fruit rot toxins from the fungus caused Macrphoma fruit rot of apple. Agric. Biol. Chem. 1983, 47, 1155–1157. [Google Scholar]
- Sassa, T.; Uchie, K.; Kato, H.; Onuma, Y. Decomposition of fruit rot toxin A, a host-selective phytotoxin from Botryosphaeria berengeriana. Agric. Biol. Chem. 1987, 51, 271–272. [Google Scholar] [CrossRef] [Green Version]
- Loesgen, S.; Bruhn, T.; Meindl, K.; Dix, I.; Schulz, B.; Zeeck, A.; Bringmann, G. (+)-Flavipucine, the missing member of the pyridione epoxide family of fungal antibiotics. Eur. J. Org. Chem. 2011, 2011, 5156–5162. [Google Scholar] [CrossRef]
- Evidente, A.; Fiore, M.; Bruno, G.; Sparapano, L.; Motta, A. Chemical and biological characterisation of sapinopyridione, a phytotoxic 3,3,6-trisubstituted-2,4-pyridione produced by Sphaeropsis sapinea, a toxigenic pathogen of native and exotic conifers, and its derivatives. Phytochemistry 2006, 67, 1019–1028. [Google Scholar] [CrossRef]
- Grandolini, G.; Casinovi, C.G.; Radics, L. On the biosynthesis of flavipucine. J. Antibiot. 1987, 40, 1339–1340. [Google Scholar] [CrossRef] [Green Version]
- Findlay, J.A.; Krepinsky, J.; Shum, A.; Casinovi, C.G.; Radics, L. The structure of isoflavipucine. Can. J. Chem. 1977, 55, 600–603. [Google Scholar] [CrossRef]
- Kakeya, H.; Takahashi, I.; Okada, G.; Isono, K.; Osada, H. Epolactaene, a novel neuritogenic compound in human neuroblastoma cells, produced by a marine fungus. J. Antibiot. 1995, 48, 733–735. [Google Scholar] [CrossRef] [Green Version]
- Weber, D.; Gorzalczany, S.; Martino, V.; Acevedo, C.; Sterner, O.; Anke, T. Metabolites from endophytes of the medicinal plant Erythrina crista-galli. Z. Naturforsch. C J. Biosci. 2005, 60, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.S.; Li, X.M.; Du, F.Y.; Li, C.S.; Proksch, P.; Wang, B.G. Secondary metabolites from a marine-derived endophytic fungus Penicillium chrysogenum QEN-24S. Mar. Drugs 2010, 9, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Yanga, X.; Liu, Y.; Li, S.; Yang, F.; Zhao, L.; Peng, L.; Ding, Z. Antimicrobial metabolites from endophytic Streptomyces sp. YIM61470. Nat. Prod. Commun. 2014, 9, 1287–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagas, F.O.; Caraballo-Rodríguez, A.M.; Dorrestein, P.C.; Pupo, M.T. Expanding the chemical repertoire of the endophyte Streptomyces albospinus RLe7 reveals amphotericin B as an inducer of a fungal phenotype. J. Nat. Prod. 2017, 80, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Kleigrewe, K.; Aydin, F.; Hogrefe, K.; Piecuch, P.; Bergander, K.; Würthwein, E.U.; Humpf, H.U. Structure elucidation of new fusarins revealing insights in the rearrangement mechanisms of the Fusarium mycotoxin fusarin C. J. Agric. Food Chem. 2012, 60, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Kakeya, H.; Kageyama, S.; Nie, L.; Onose, R.; Okada, G.; Beppu, T.; Norbury, C.J.; Osada, H. Lucilactaene, a new cell cycle inhibitor in p53-transfected cancer cells, produced by a Fusarium sp. J Antibiot. 2001, 54, 850–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S.; Motoyama, T.; Futamura, Y.; Uramoto, M.; Nogawa, T.; Hayashi, T.; Hirota, H.; Tanaka, A.; Takahashi-Ando, N.; Kamakura, T.; et al. Biosynthetic gene cluster identification and biological activity of lucilactaene from Fusarium sp. RK97-94. Biosci. Biotechnol. Biochem. 2020, 84, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Kakeya, H.; Uno, T.; Shoji, M.; Osada, H.; Hayashi, Y. Determination by asymmetric total synthesis of the absolute configuration of lucilactaene, a cell-cycle inhibitor in p53-transfected cancer cells. Angewandte Chemie Int. Ed. Engl. 2005, 44, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, L.; Wang, Y.; Wang, W.; Hao, J.; Zhu, W. Bioactive natural products of Aspergillus sp. OUCMDZ-1914, an aciduric fungus from the mangrove soils. Chin. J. Org. Chem. 2015, 35, 1955–1960. [Google Scholar] [CrossRef] [Green Version]
- Nichols, M.A.; Waner, M.J. Kinetic and mechanistic studies of the deuterium exchange in classical keto-enol tautomeric equilibrium reactions. J. Chem. Educ. 2010, 87, 952–955. [Google Scholar] [CrossRef]
- Kusakabe, Y.; Mizutani, S.; Kamo, S.; Yoshimoto, T.; Tomoshige, S.; Kawasaki, T.; Takasawa, R.; Tsubaki, K.; Kuramochi, K. Synthesis, antibacterial and cytotoxic evaluation of flavipucine and its derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1390–1394. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Bharate, S.B.; Khan, S.I.; Yunus, N.A.; Chauthe, S.K.; Jacob, M.R.; Tekwani, B.L.; Khan, I.A.; Singh, I.P. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg. Med. Chem. 2007, 15, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]

 ) and COSY (
) and COSY (  ) correlations of 4 ROESY (
) correlations of 4 ROESY (  ) correlations of 4.
) correlations of 4.


| Sapinopyridione [25] | Compound 1 | (-)-Flavipucine [22,27] | Compound 2 | |||||
|---|---|---|---|---|---|---|---|---|
| C | δC | δH | δCa | δHb (J in Hz) | δC | δH | δCa | δHb (J in Hz) |
| 2 | 68.6 | 3.94, s | 68.7 | 3.94, s | 68.8 | 3.86, s | 68.8 | 3.84, s |
| 3 | 59.9 | 60.0 | 60.0 | 59.8 | ||||
| 4 | 168.2 | 168.7 | 168.8 | 168.6 | ||||
| 6 | 154.9 | 155.8 | 156.2 | 155.8 | ||||
| 7 | 107.1 | 5.64, s | 107.2 | 5.63, s | 107.3 | 5.64, m | 107.2 | 5.63, s |
| 8 | 186.2 | 186.5 | 186.9 | 186.4 | ||||
| 9 | 206.5 | 206.6 | 203.6 | 203.7 | ||||
| 10 | 44.3 | 3.28, ddq | 44.5 | 3.22, m | 49.6 | 2.68, m | 49.6 | 2.62, dd (16.7, 7.2) 2.68, dd (16.7, 7.2) |
| 11 | 25.3 | 1.79, ddq | 25.4 | 1.78, m | 24.1 | 2.1, m | 24.0 | 2.17 c |
| 1.52, ddq | 1.50, m | |||||||
| 12 | 11.1 | 0.97, t | 11.2 | 0.94, t (7.4) | 22.5 | 0.94, d (6.6) | 22.6 | 0.92, d ( 6.7) |
| 13 | 12.6 | 1.05, d | 12.7 | 1.02, d (6.6) | 22.8 | 0.98, d (6.6) | 22.9 | 0.96, d, (6.7) |
| 14 | 20.8 | 2.19, s | 20.8 | 2.16, s | 20.8 | 2.18, d (1.0) | 20.8 | 2.16, s |
| NH | 8.38, br s | 9.24, br s | 9.14, br s | 9.22, br s | ||||
| 3 (CDCl3) | 4 (CDCl3:methanol-d4 4:1) | |||
|---|---|---|---|---|
| δCa | δHb (J in Hz) | δCa | δHb (J in Hz) | |
| 1 | 15.9 | 1.72, d (7.1) | 15.8 | 1.55, d (7.1) |
| 2 | 139.9 | 6.96, q (7.1) | 140.1 | 6.76, q (7.1) |
| 3 | 130.3 | 130.4 | ||
| 4 | 123.1 | 5.97, br s | 122.7 | 5.76, s |
| 5 | 137.7 | 138.0 | ||
| 6 | 135.6 | 6.23, d (15.6) | 135.0 | 6.10, d (15.6) |
| 7 | 128.2 | 5.68, dt (15.2, 6.6) | 128.8 | 5.60, dt (15.4, 6.9) |
| 8 | 31.5 | 2.32, m | 31.6 | 2.20, m |
| 9 | 28.4 | 2.45, m | 29.4 | 2.30, m |
| 10 | 148.6 | 6.56, t (6.4) | 146.6 | 6.59, t (4.6) |
| 11 | 137.8 | 138.4 | ||
| 12 | 191.4 | 196.2 | ||
| 13 | 139.1 | 50.0 | 3.21, s | |
| 14 | 149.5 | 6.83, s | 76.5 | 4.29, s |
| 15 | 89.2 | 83.9 | ||
| 17 | 168.3 | 168.3 | ||
| 18 | 24.8 | 1.61, s | 20.3 | 1.46, s |
| 19 | 167.8 | 168.3 | ||
| 20 | 51.9 | 3.74, s | 51.9 | 3.55, s |
| 21 | 14.3 | 1.62, s | 14.3 | 1.45, s |
| 22 | 11.1 | 1.89, s | 11.6 | 1.67, s |
| 23 | 50.8 | 3.18, s | 60.7 | 3.45, 3.49, m |
| 24 | 70.4 | 3.65, m | ||
| 25 | 61.9 | 3.39, 3.58, m c | ||
| Compound | Lettuce | Bentgrass |
|---|---|---|
| EtOAc extract | no growth | no growth |
| 3:1 mixture of 1 and 2 | no growth | no growth |
| Compound | Chloroquine-Sensitive (D6)-Clone | Chloroquine-Resistant (W2)-Clone | Cytotoxicity to Vero Cells | ||
|---|---|---|---|---|---|
| IC50 µg/mL (µM) | SI | IC50 µg/mL (µM) | SI | IC50 µg/mL (µM) | |
| 3:1 mixture of 1 and 2 | NA | NA | NC | ||
| 3 | NA | NA | NC | ||
| Fraction containing 4 a | <0.523 (<1.17) | >9 | <0.523 (<1.17) | >9 | NC |
| Chloroquine b | 0.016 (0.03) | 496.6 | 0.16 (0.31) | 48.1 | NC |
| Artemisinin b | 0.0056 (0.02) | 845 | 0.003 (0.01) | 1690 | NC |
| Compound | SK-MEL | KB | BT-549 | SK-OV-3 | LLC-PK11 |
|---|---|---|---|---|---|
| 3:1 mixture of 1 and 2 | 5.05 | 21.9 | 20.3 | 6.7 | 7.6 |
| Doxorubicin a | 1.29 | 2.12 | 1.83 | 1.47 | 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumarihamy, M.; Rosa, L.H.; Techen, N.; Ferreira, D.; Croom, E.M., Jr.; Duke, S.O.; Tekwani, B.L.; Khan, S.; Nanayakkara, N.P.D. Antimalarials and Phytotoxins from Botryosphaeria dothidea Identified from a Seed of Diseased Torreya taxifolia. Molecules 2021, 26, 59. https://doi.org/10.3390/molecules26010059
Kumarihamy M, Rosa LH, Techen N, Ferreira D, Croom EM Jr., Duke SO, Tekwani BL, Khan S, Nanayakkara NPD. Antimalarials and Phytotoxins from Botryosphaeria dothidea Identified from a Seed of Diseased Torreya taxifolia. Molecules. 2021; 26(1):59. https://doi.org/10.3390/molecules26010059
Chicago/Turabian StyleKumarihamy, Mallika, Luiz H. Rosa, Natascha Techen, Daneel Ferreira, Edward M. Croom, Jr., Stephen O. Duke, Babu L. Tekwani, Shabana Khan, and N. P. Dhammika Nanayakkara. 2021. "Antimalarials and Phytotoxins from Botryosphaeria dothidea Identified from a Seed of Diseased Torreya taxifolia" Molecules 26, no. 1: 59. https://doi.org/10.3390/molecules26010059