Detection, Purification and Elucidation of Chemical Structure and Antiproliferative Activity of Taxol Produced by Penicillium chrysogenum
Abstract
:1. Introduction
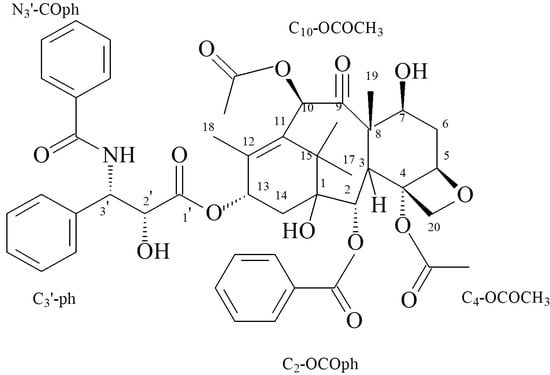
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation and Characterization of Fungi Suspected to Produce the Taxol Molecule
4.2. Determination of the Taxol Substance in Cell Free Supernatant (CFS) of P. chrysogenum
4.3. Detection of Genes Encoding Taxol Production within P. chrysognum Genome
4.4. Optimization of Growth Conditions Necessary for Taxol Production by P. chrysogenum
4.5. High Performance Liquid Chromatography (HPLC) of P. chrysogenum Taxol
4.6. Instrumental Analysis of the Suspected Taxol Substance
4.7. Antiproliferative Activity of the Taxol Substance Produced by P. chrysogenum
4.8. Statistical Analyzes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enan, G. Inhibition of Clostridium perfringens LMG11264 in Meat Samples of Chicken Turkey and Beef by the Bacteriocin Plantaricin UG1. Int. J. Poult. Sci. 2006, 5, 195–200. [Google Scholar]
- Enan, G.; Abdel Shafi, S.; Abdel-Haliem, M.F.; Negm, S. Characterization of probiotic lactic acid bacteria to be used as starter and protective cultures for dairy fermentations. Int. J. Probiotics Prebiotics 2013, 8, 157–163. [Google Scholar]
- Enan, G.; Al-Mohammadi, A.-R.; El-Didamony, G.; Abdel-halelie, M.E.F.; Zakaria, A. Antimicrobial activity of Enterococcusfaecium NM2 Isolated from Urine: Purification, Characterization and Bactericidal Action of Enterocin NM2. Asian J. Appl. Sci. 2014, 7, 621–634. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafi, S.; Osman, A.; Enan, G.; Sitohy, M.Z. Antibacterial activity of methylated eggwhite proteins against pathogenic G+andG− bacteria matching antibiotics. Springer Plus 2016, 5, 983–996. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.; El-Didamony, G.; Sitohy, M.; Khalifa, M.; Enan, G. Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int. J. Appl. Res. Nat. Prod. 2016, 9, 17–28. [Google Scholar]
- Enan, G.; Osman, M.E.; Abdel–Haliem, M.E.F.; Abdel-Ghany, S. Advances in microbial and nucleic acids biotechnology. Biomed. Res. Intern. 2018, 2018, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Abdel Shafi, S.; Al-Mohammadi, A.R.; Osman, A.; Enan, G.; Abdel Hameid, S.; Sitohy, M. Characterization and antibacterial activity of 7S and 11S globulins isolated from cowpea seed protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafi, S.; Al-Mohammadi, A.R.; Almanaa, T.N.; Moustafa, A.H.; Saad, T.M.M.; Ghonemy, A.; Anacarso, I.; Enan, G.; El-Gazzar, N. Identification and testing antidermatophytic oxaborole-6-benzenesulphonoamide derivative (OXBS) from Streptomyces atrovirens KM192347 isolated from soil. Antibiotics 2020, 9, 176. [Google Scholar] [CrossRef]
- El-Gazzar, N.S.; Enan, G. Advances in Phage Inspired Nanosciencebased Therapy. Nanobioscience; Saxena, S.K., Khurana, S.P., Eds.; Springer Nature Singapore PteLtd.: Singapore, 2020. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Muthumary, J.; Hur, B.K. Production of taxol from Phyllostictaspi-narum, an endophytic fungus of Cupressus sp. Eng. Life Sci. 2008, 8, 438–446. [Google Scholar] [CrossRef]
- Pimentel, G.; Molina, A.P.; Dionisio, M.R.; Marostica, J.R.; Pastore, G.M. The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol. Res. Int. 2011, 2011, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.; Liu, S.; Nie, Y. Anticancer activity of chamae jasmine: Effect on tubulin protein. Molecules 2011, 16, 6243–6254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangadevi, V.; Muthumary, J. Taxol production by Pestalotiopsis terminaliae, an endophytic fungus of Terminaliaarjuna (arjuntree). Biotechnol. Appl. Biochem. 2009, 52, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, L.; Liu, J.; Lin, J.; Tang, K. A review: Recent advances and future prospects of taxol-producing endophytic fungi. Appl. Microbiol. Biotechnol. 2010, 86, 1707–1717. [Google Scholar] [CrossRef]
- Mohan Pandi, R.; Senthil, K.; Yong-Keun, C.; Hyung, J.K.; Johnpaul, M. Isolation and detection of taxol, an anticancer drug produced from Lasiodiplodiatheobromae, an endophytic fungus of the medicinal plant. Afr. J. Biotechnol. 2011, 10, 1428–1435. [Google Scholar]
- Malik, S.; Cusido, R.M.; Mirjalili, M.H.; Moyano, E.J.; Palazon, J.; Bonfill, M. Taxol from Corylusavellana: Paving the way for a new source of this anti-cancer drug. Process Biochem. 2011, 46, 23–34. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Khalaf, S.A.; Abdel Hamid, G.; El-Batrik, M.I. Screening, morphological and molecular identification of cystathionine γ-lyase producing fungi. ActaBiol. Hung. 2015, 66, 119–132. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Tao, W. Preliminary study on fermentation conditions of taxol-producing endophytic fungus. Chem. Ind. Eng. Prog. 2008, 27, 883–886. [Google Scholar]
- Trivedi, P.C. Medicinal Plants: Traditional Knowledge; I.K. International Pvt Ltd.: New Delhi, India, 2006. [Google Scholar]
- Chen, J.H.; Liu, J.J.; Zang, G.G.; Li, Y.J.; Zhao, L.N. Screening of taxol-producing endophytic fungi and regulation of fermentation conditions. J. Central South Univ. 2004, 35, 65–69. [Google Scholar]
- Li, J.Y.; Strobel, G.; Sidhu, R.; Hess, W.M.; Ford, E.J. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiology 1996, 142, 2223–2226. [Google Scholar] [CrossRef] [Green Version]
- Strobel, G.; Yang, X.; Sears, J.; Kramer, R.; Sidhu, R.S.; Hess, W.M.; Young, B. Endophytic fungus of Taxus wallachiana. Microbiology 1996, 142, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Kebeish, M.R.; E-Sayed, A.S.A.; Fahmy, H.; Abdel-Ghany, A. Molecular cloning, biochemical characterization and antitumor properties of a novel L-asparaginase from Synechococcus elongates. Biochemistry 2016, 81, 1173–1181. [Google Scholar] [PubMed]
- Zhang, P.; Zhou, P.P.; Yu, L.-J. Anendophytictaxol-producing fungus from Taxus Aspergillus candidus MD3. FEMS Microbiol. Lett. 2009, 293, 155–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, K.; Croteau, R. Taxol biosynthetic genes. Phytochemistry 2001, 58, 107. [Google Scholar] [CrossRef]
- Deng, B.W.; Liu, K.H.; Chen, W.Q.; Ding, X.W.; Xie, X.C. Fusarium solani Tax-3, an ewendophytic taxol-producing fungus from Taxus chinensis. World J. Microbiol. Biotechnol. 2009, 25, 139–143. [Google Scholar] [CrossRef]
- Ali, G.S.; Norman, D.; El-Sayed, A.S.A. Soluble and volatile metabolites of plant growth promoting Rhizobacteria (PGPRs). Role and Practical applications ininhibiting pathogens activating induced Systemic Resistance (ISR). Adv. Bot. Res. 2015, 75, 241–284. [Google Scholar]
- Garyali, S.; Kumar, A.; Redy, M.S. Enhancement of taxol production from endophytic fungus Fusariumredolens. Biotechnol. Bioprocess Eng. 2014, 19, 908–915. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Shindia, A.A.; Abou-Zaid, A.A.; Yassin, A.M. Aspergillus nidulans argininedeiminase - Dextran conjugates with enhanced molecular stability, proteolytic resistance, pharmacokinetic properties and anticancer activity. Enzym. Microb. Technol. 2019, 131, 109432. [Google Scholar] [CrossRef]
- Somjaipeng, S.; Medina, A.; Kwasna, H.; Ortiz, J.O.; Magan, N. Isolation, identification, and ecology of growth and taxol production by an endophytic strain of Paraconiothyrium variabile from English yew trees (Taxusbaccata). Fungal Biol. 2015, 119, 1022–1031. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Iqrar, I.; Ali, R.; Norman, D.; Brennan, M.; Ali, G.S. A glucanolytic Pseudomonas sp. Associated with Smilaxbona-nox L. displays strong activity against Phytophthora parasitica. Microbiol. Res. 2018, 207, 140–152. [Google Scholar] [CrossRef]
- Li, G.; Chun-Qiang, T.; Shu-Shen, Y. Optimization of Aspergillus fumigates TMS-26 taxol production fermentation system by precursors, elicitors and fermentation conditions. Mycosystema 2015, 34, 1165–1175. [Google Scholar]
- El-Sayed, A.S.A.; Mohamed, N.M. Restoring the Biosynthetic Machinery of Taxol of Aspergillus terreus via cocultivation with the endophytic microbiome of Podocarpus gracilior. Pilger. Sci. Rep. 2019, 9, 11534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staniek, A.; Woerdenbag, H.; Kayser, O. Taxomyces andreanae: A presumed paclitaxel producer demystified? Planta Med. 2009, 75, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Safan, S.; Mohamed, N.Z.; Shaban, L.; Ali, G.S.; Sitohy, M.Z. Induction of Taxol biosynthesis by Aspergillus terreus, endophyte of Podocarpus gracilior Pilgerupon intimate interaction with the plant endogenous microbes. Process Biochem. 2018, 71, 31–40. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Ali, D.M.I.; Yassin, M.A.; Zayed, R.W.; Ali, G.S. Sterolinhibitor “Fluconazole” enhance the Taxol yield and molecular expression of its encoding genes cluster from Aspergillus flavipes. Process. Biochem. 2019, 76, 55–67. [Google Scholar] [CrossRef]
- El-Sayed, A.S.A.; Shindia, A.A.; AbouZaid, A.A.; Yassin, A.M.; Ali, G.S.; Sitohy, M. Biochemical characterization of peptidyl arginin deiminase-like orthologs from thermotolerant Emericella dentata and Aspergillus nidulans. Enzym. Microb. Technol. 2019, 124, 41–53. [Google Scholar] [CrossRef]
- Chmurny, G.N.; Hilton, B.D.; Brobst, S.; Look, S.A.; Witherup, K.M.; Beutler, J.A. 1H- and 13C- nmr assignments for taxol, 7-epi-taxol, and cephalomannine. J. Nat. Prod. 1992, 55, 414–423. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almaary, K.H.; Ismail, A.; Polizzi, G. Influence of Funneliformis mosseae enhanced with titanium dioxide nanoparticles (TiO2NPs) on Phaseolus vulgaris L. Under salinity stress. PLoS ONE 2020, 15, e0235355. [Google Scholar] [CrossRef]
- Das, A.; Rahman, M.I.; Ferdous, A.S.; Amin, A.; Rahman, M.; Nahar, N.; Uddin, A.; Islam, M.R.; Khan, H. An endophytic Basidiomycete, Grammothelelineata, isolated from Corchorusolitorius, produces paclitaxel that shows cytotoxicity. PLoS ONE 2017, 2017, e0178612. [Google Scholar]
- El-Gazzar, N.; Ismail, A.M. The potential use of Titanium, Silver and Selenium nanoparticles in controlling leaf blight of tomato caused by Alternaria alternata. Biocatal. Agric. Biotechnol. 2020, 27, 101708. [Google Scholar] [CrossRef]
- Selim, S.; Al Jaouni, S. Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm, BMC Complement. Altern. Med. 2015, 15, 301. [Google Scholar]
- Moubasher, A.H. Soil Fungi in Qatar and Other Arab Countries; The Centre for Scientific and Applied Research: Doha, Qatar, 1993. [Google Scholar]
- Abdel-Salam, H.A.; El-Khamisssy, T.; Enan, G.A.; Hollenberg, C.P. Expression of mouse anticreatine kinase (MAK33) monoclonal antibody in the yeast Hansenula polymorpha. Appl. Microbiol. Biotechnol. 2001, 56, 157–164. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Abdel-Azim, S.; Ibrahim, H.; Yassin, M.A.; Abdel-Ghany, S.; Esener, S.; Ali, G.S. Biochemical stability and molecular dynamic characterization of Aspergillus fumigates cystathionine γ-Lyasein response to various reaction effectors. Enzym. Microb. Technol. 2015, 81, 31–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Shafi, S.; Al-Mohammadi, A.; Hamdi, S.; Moustafa, A.H.; Enan, G. Biological characterization and inhibition of Streptococcus pyogenes ZUH1 causing chronic cystitis by both Crocus sativus methanol extract; bee honey singly or in combination with antibiotics: Aninvitrostudy. Molecules 2019, 24, 2903. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.S.A.; ElSayed, M.T.; Rady, A.; Zein, N.; Enan, G.; Shindia, A.A.; El-Hefnawy, S.A.; Sitohy, M.; Sitohy, B. Exploiting the biosynthetic potency of Taxol from fungal endophytes of Conifers plants; Genome mining, and metabolic manipulation. Molecules 2020, 25, 3000. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Nada, H.M.; Hassan, M.N. Purification, immobilization, and biochemical characterization of L-arginine deiminase from thermophilic Aspergillus fumigatus KJ434941: AnticancerActivity In vitro. Biotechnol. Prog. 2015, 31, 396–405. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.S.A.; Maher, F.; Marwa, A.Y.; Nabila, Z.; Shaima, M.; Mahmoud, S.; Basel, S. Conjugation of Aspergillus flavipes Taxol with Porphyrin increases the anticancer activity of Taxol and ameliorates its cytotoxic effects. Molecules 2020, 25, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Z.Q.; Yang, Y.Y.; Zhao, N.; Wang, Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojapyew Taxusxmedia. BMC Microbiol. 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nims, E.; Dubois, C.P.; Roberts, S.C.; Walker, E.L. Expression profiling of genes involved in paclitaxel biosynthesis for targeted metabolic engineering. Metab. Eng. 2006, 8, 385–394. [Google Scholar] [CrossRef]
- Zhao, K.; Ping, W.; Li, Q.; Hao, S.; Zhao, L.; Gao, T.; Zhou, D. Aspergillus niger var. taxi, a new species variant of taxol-producing fungus isolated from Taxuscuspidatain China. J. Appl. Microbiol. 2009, 107, 1202–1207. [Google Scholar] [CrossRef]
- Gangadevi, V.; Muthumary, J. Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aeglemarmelos Correaex Roxb. World J. Microbiol. Biotechnol. 2008, 24, 717–724. [Google Scholar] [CrossRef]
- Sun, D.; Ran, X.; Wang, J. Isolation and identification of a taxol-producing endophytic fungus from Podocarpus. Acta Microbiol. Sin. 2008, 48, 589–595. [Google Scholar]
- El-Sayed, A.S.A.; Abdel-Ghany, S.E.; Ali, G.S. Genome editing approaches: Manipulating of lovastatin and taxol synthesis of filamentous fungi by CRISPR/Cas9system. Appl. Microbiol. Biotechnol. 2017, 101, 3953–3976. [Google Scholar] [CrossRef] [PubMed]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetra-zolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.G.D.; Torri, J.H. Principles and Procedures of Statistics; Mc Graw-Hill: New York, NY, USA, 1960. [Google Scholar]









| Value Applied | Taxol µg/L | |
|---|---|---|
| 4 | 0.0 | |
| 6 | 50 h ± 4.0 | |
| 8 | 85 g ± 5.0 | |
| 10 | 110 f ± 10.0 | |
| 12 | 150 d ± 5.0 | |
| 14 | 170 c ± 5.0 | |
| 16 | 187 bc ± 5.0 | |
| 18 | 200 a ± 20.0 | |
| 20 | 195 b ± 5.0 | |
| 22 | 187 bc ±0.0 | |
| 24 | 160 d ± 2.0 | |
| 26 | 130 e ± 10.0 | |
| 28 | 80 gh ± 10.0 | |
| 30 | 40 i ± 5.0 | |
| pH Value | 2 | 0.00 |
| 3 | 20 f ± 2.0 | |
| 4 | 65 e ± 3.5 | |
| 5 | 100 d ± 1.0 | |
| 6 | 150 c ± 5.0 | |
| 7 | 200 b ± 2.0 | |
| 8 | 220 a ± 5.0 | |
| 9 | 95 d ± 5.0 | |
| Incubation Temperature (°C) | 20 | 100.31 c ± 0.0 |
| 25 | 150.43 c ± 10.0 | |
| 30 | 230.92 a ± 0.0 | |
| 35 | 200.1 b ± 10.0 | |
| 40 | 60.86 d ± 0.0 | |
| Agitation Speeds (rpm) | Static | 150.47 c ± 0.0 |
| 90 | 200.27 bc ± 10.0 | |
| 120 | 250.21 a ± 5.0 | |
| 150 | 210.51 a ± 0.0 | |
| 200 | 200.93 ab ± 10.0 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sayed, A.; Enan, G.; Al-Mohammadi, A.-R.; Moustafa, A.H.; El-Gazzar, N. Detection, Purification and Elucidation of Chemical Structure and Antiproliferative Activity of Taxol Produced by Penicillium chrysogenum. Molecules 2020, 25, 4822. https://doi.org/10.3390/molecules25204822
El-Sayed A, Enan G, Al-Mohammadi A-R, Moustafa AH, El-Gazzar N. Detection, Purification and Elucidation of Chemical Structure and Antiproliferative Activity of Taxol Produced by Penicillium chrysogenum. Molecules. 2020; 25(20):4822. https://doi.org/10.3390/molecules25204822
Chicago/Turabian StyleEl-Sayed, Ashraf, Gamal Enan, Abdul-Raouf Al-Mohammadi, Ahmed H. Moustafa, and Nashwa El-Gazzar. 2020. "Detection, Purification and Elucidation of Chemical Structure and Antiproliferative Activity of Taxol Produced by Penicillium chrysogenum" Molecules 25, no. 20: 4822. https://doi.org/10.3390/molecules25204822