Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation
Abstract
:1. Introduction
2. Results and Discussion
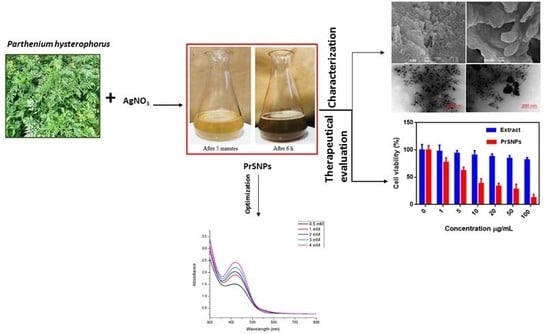
2.1. Visual Inspection
2.2. Phytochemical Screening
2.3. UV-Visible Spectroscopy
2.3.1. Effect of Reaction Time
2.3.2. Effect of Silver Nitrate Concentration
2.3.3. Effect of Leaf Extract Concentration
2.4. DLS and Zeta Potential (Z.P) Analysis
2.5. SEM Investigation
2.6. TEM Analysis
2.7. FTIR Analysis
2.8. TGA and DSC Studies
2.9. Antimicrobial Activity of Biogenic PrSNPs
2.9.1. Determination of MIC and MBC for Microbial Growth
2.9.2. Anti-Inflammatory Activity of PrSNPs
2.10. In Vitro Antioxidant Assay
2.10.1. DPPH Assay
2.10.2. Hydrogen Peroxide (H2O2) Scavenging Assay
2.10.3. Nitric Oxide (NO) Radical Scavenging Assay
2.11. In Vitro Cytotoxicity Assay
2.12. Treatment of Wastewater by PrSNPs
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Leaf Extract
3.2.2. Phytochemical Analysis
Mayer’s Test (Alkaloids)
Molisch’s Test (Carbohydrates and Glycosides)
Biuret Test (Proteins)
Ninhydrin Test (Amino acid)
Ferric Chloride Test (Phenols)
Neutral Ferric Chloride Test (Tannins)
Shinoda Test (Flavonoids)
Salkowski’s Test (Terpenoids)
Froth Test (Saponins)
3.3. Phytoreduction of Parthenium Silver Nanoparticles (PrSNPs)
3.4. Visual Inspection
3.5. UV-Visible Spectroscopy
3.6. Dynamic Light Scattering (DLS) and Zeta Potential (Z.P) Analysis
3.7. Scanning Electron Microscopy (SEM)
3.8. Transmission Electron Microscopy (TEM)
3.9. Fourier Transform Infrared Spectroscopy (FTIR)
3.10. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.11. Antimicrobial Activity of Biogenic PrSNPs
Determination of Minimum Inhibitory Concentration (MIC) and Minimum Biocidal Concentration (MBC) for Microbial Growth
3.12. In Vitro Anti-Inflammatory Assay
3.13. In Vitro Antioxidant Assay
3.13.1. DPPH (2,2-diphenyl-2-picrylhydrazyl) Assay
3.13.2. Hydrogen Peroxide (H2O2) Assay
3.13.3. Nitric oxide (NO) Radical Scavenging Assay
3.14. In Vitro Cytotoxicity Assay
3.15. Treatment of Water by PrSNPs
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ingale, A.G.; Chaudhari, A. Biogenic synthesis of nanoparticles and potential applications: An eco-friendly approach. J. Nanomed. Nanotechol. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Hebbalalu, D.; Lalley, J.; Nadagouda, M.N.; Varma, R.S. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustain. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Mohammadlou, M.; Maghsoudi, H.; Jafarizadeh-Malmiri, H. A review on green silver nanoparticles based on plants: Synthesis, potential applications and eco-friendly approach. Int. Food Res. J. 2016, 23. [Google Scholar]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Soni, M.; Mehta, P.; Soni, A.; Goswami, G.K. Green nanoparticles: Synthesis and applications. IOSR J. Biotechnol. Biochem. 2018, 4, 78–83. [Google Scholar]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E.; Ferdov, S.; Singh, M.P. Silver nanoparticles: Synthesis, properties, and applications. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraj, C.; Jagan, E.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- AshaRani, P.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Vasanth, K.; Ilango, K.; MohanKumar, R.; Agrawal, A.; Dubey, G.P. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Gengan, R.; Anand, K.; Phulukdaree, A.; Chuturgoon, A. A549 lung cell line activity of biosynthesized silver nanoparticles using Albizia adianthifolia leaf. Colloids Surf. B Biointerfaces 2013, 105, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Farah, M.A.; Ali, M.A.; Chen, S.-M.; Li, Y.; Al-Hemaid, F.M.; Abou-Tarboush, F.M.; Al-Anazi, K.M.; Lee, J. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf. B Biointerfaces 2016, 141, 158–169. [Google Scholar] [CrossRef]
- Farias, C.B.; Ferreira Silva, A.; Diniz Rufino, R.; Moura Luna, J.; Gomes Souza, J.E.; Sarubbo, L.A. Synthesis of silver nanoparticles using a biosurfactant produced in low-cost medium as stabilizing agent. Electron. J. Biotechnol. 2014, 17, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Van Dong, P.; Ha, C.H.; Kasbohm, J. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int. Nano Lett. 2012, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Mater. Met. Eng. 2008, 2, 91–98. [Google Scholar]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An overview of hydrogels and their role in transdermal drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–11. [Google Scholar] [CrossRef]
- Montes-García, V.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Metal nanoparticles and supramolecular macrocycles: A tale of synergy. Chem. Eur. J. 2014, 20, 10874–10883. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Ying, J.Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef]
- Castro, L.; Blázquez, M.L.; González, F.G.; Ballester, A. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev. Adv. Sci. Eng. 2014, 3, 199–216. [Google Scholar] [CrossRef]
- Vamanu, E.; Ene, M.; Biță, B.; Ionescu, C.; Crăciun, L.; Sârbu, I. In vitro human microbiota response to exposure to silver nanoparticles biosynthesized with mushroom extract. Nutrients 2018, 10, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajwa, A.A.; Chauhan, B.S.; Farooq, M.; Shabbir, A.; Adkins, S.W. What do we really know about alien plant invasion? A review of the invasion mechanism of one of the world’s worst weeds. Planta 2016, 244, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Harmful and beneficial aspects of Parthenium hysterophorus: An update. 3 Biotech. 2011, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Adkins, S.W.; McClay, A.; Bajwa, A.A.; Shabbir, A.; Dhileepan, K. Biology and ecology. In Parthenium Weed: Biology, Ecology and Management; CABI: Oxfordshire, UK, 2018; pp. 7–39. [Google Scholar]
- Das, B.; Reddy, V.S.; Krishnaiah, M.; Sharma, A.; Kumar, K.R.; Rao, J.V.; Sridhar, V. Acetylated pseudoguaianolides from Parthenium hysterophorus and their cytotoxic activity. Phytochemistry 2007, 68, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Fazal, H.; Ahmad, N.; Ullah, I.; Inayat, H.; Khan, L.; Abbasi, B.H. Antibacterial potential in Parthenium hysterophorus, Stevia rebaudiana and Ginkgo biloba. Pak. J. Bot. 2011, 43, 1307–1313. [Google Scholar]
- Bezuneh, T.T. Phytochemistry and antimicrobial activity of Parthenium hysterophorus L.: A review. Sci. J. Anal. Chem. 2015, 3, 30–38. [Google Scholar] [CrossRef]
- Parashar, V.; Parashar, R.; Sharma, B.; Pandey, A.C. Parthenium leaf extract mediated synthesis of silver nanoparticles: A novel approach towards weed utilization. Dig. J. Nanomater. Biostruct. (DJNB) 2009, 4, 45–50. [Google Scholar]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. 2018, 25, 10328–10339. [Google Scholar] [CrossRef]
- Bendezú Ccanto, J.Y. Efecto de la Germinación de Tres Variedades de Quinua: Roja (INIA-415 Pasankalla), Negra (INIA 420-Negra Collana) y Blanca (Salcedo INIA) en la Formulación y Elaboración de una Bebida Funcional con Capacidad Antioxidante. Available online: https://hdl.handle.net/20.500.12672/10085 (accessed on 20 July 2020).
- Erdoğan, T.; Yılmaz, F.F.; Kıvçak, B.; Özyazıcı, M. Green synthesis of silver nanoparticles using Arbutus andrachne leaf extract and its antimicrobial activity. Trop. J. Pharm. Res. 2016, 15, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Khan, T.; Ullah, N.; Khan, M.A.; Nadhman, A. Plant-based gold nanoparticles; a comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications. Adv. Colloid Interface Sci. 2019, 272, 102017. [Google Scholar] [CrossRef] [PubMed]
- DESHPANDE, B.; SHARMA, D.; PANDEY, B. PHYTOCHEMICALS AND ANTIBACTERIAL SCREENING OF Parthenium hysterophorus. Indian J. Sci. Res. 2017, 13, 199–202. [Google Scholar]
- Siddiqui, S.; Verma, A.; Rather, A.A.; Jabeen, F.; Meghvansi, M.K. Preliminary phytochemicals analysis of some important medicinal and aromatic plants. Adv. Biol. Res. 2009, 3, 188–195. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prathna, T.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf. B Biointerfaces 2011, 82, 152–159. [Google Scholar] [CrossRef]
- Vanaja, M.; Paulkumar, K.; Gnanajobitha, G.; Rajeshkumar, S.; Malarkodi, C.; Annadurai, G. Herbal plant synthesis of antibacterial silver nanoparticles by Solanum trilobatum and its characterization. Int. J. Met. 2014, 2014. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. A facile and rapid method for the black pepper leaf mediated green synthesis of silver nanoparticles and the antimicrobial study. Appl. Nanosci. 2014, 4, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Zolfaghari, B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Gorai, S. Bio-based Synthesis and Applications of SnO2 Nanoparticles-An Overview. J. Mater. Environ. Sci. 2018, 9, 2894–2903. [Google Scholar]
- Halawani, E.M. Rapid biosynthesis method and characterization of silver nanoparticles using Zizyphus spina christi leaf extract and their antibacterial efficacy in therapeutic application. J. Biomater. Nanobiotechnol. 2016, 8, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Umoren, S.; Obot, I.; Gasem, Z. Green synthesis and characterization of silver nanoparticles using red apple (Malus domestica) fruit extract at room temperature. J. Mater. Environ. Sci 2014, 5, 907–914. [Google Scholar]
- Ali, M.; Kim, B.; Belfield, K.D.; Norman, D.; Brennan, M.; Ali, G.S. Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract—A comprehensive study. Mater. Sci. Eng. C 2016, 58, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown Jr, G.E.; Lowry, G.V. Size-controlled dissolution of organic-coated silver nanoparticles. Environ. Sci. Technol. 2012, 46, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.P.; Das, M.K. Preparation and in vitro/in vivo evaluation of felodipine nanosuspension. Eur. J. Drug Metab. Pharmacokinet. 2014, 39, 183–193. [Google Scholar] [CrossRef]
- Sundararajan, B.; Mahendran, G.; Thamaraiselvi, R.; Kumari, B.R. Biological activities of synthesized silver nanoparticles from Cardiospermum halicacabum L. Bull. Mater. Sci. 2016, 39, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Sarkar, C.; Ghosh, C. Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Appl. Nanosci. 2015, 5, 953–959. [Google Scholar] [CrossRef]
- Kota, S.; Dumpala, P.; Anantha, R.K.; Verma, M.K.; Kandepu, S. Evaluation of therapeutic potential of the silver/silver chloride nanoparticles synthesized with the aqueous leaf extract of Rumex acetosa. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ojha, S.; Sett, A.; Bora, U. Green synthesis of silver nanoparticles by Ricinus communis var. carmencita leaf extract and its antibacterial study. Adv. Nat. Sci. Nanosci. 2017, 8, 035009. [Google Scholar] [CrossRef]
- Kalaiselvi, M.; Subbaiya, R.; Selvam, M. Synthesis and characterization of silver nanoparticles from leaf extract of Parthenium hysterophorus and its antibacterial and antioxidant activity. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 220–227. [Google Scholar]
- Wilkins, T.; Holdeman, L.V.; Abramson, I.; Moore, W. Standardized single-disc method for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob. Agents Chemother. 1972, 1, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, L.E.; Giacomelli, C.E. Stability of silver nanoparticles: Agglomeration and oxidation in biological relevant conditions. J. Nanopart. Res. 2017, 19, 156. [Google Scholar] [CrossRef]
- Rao, B.; Tang, R.-C. Green synthesis of silver nanoparticles with antibacterial activities using aqueous Eriobotrya japonica leaf extract. Adv. Nat. Sci. Nanosci. 2017, 8, 015014. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, D.K.; Mohan, S.; Gundampati, R.K.; Hasan, S.H. Photoinduced green synthesis of silver nanoparticles using aqueous extract of Physalis angulata and its antibacterial and antioxidant activity. J. Environ. Chem. Eng. 2017, 5, 744–756. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; El-Aziz, M.A.; Hanna, M.; Ahmed, M.A.; Husien, M.M.; Feng, Q. Comparative synthesis and antimicrobial action of silver nanoparticles and silver nitrate. J. Nanopart. Res. 2015, 17, 473. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Fayemi, O.E.; Ekennia, A.C.; Ebenso, E.E.; Tiedt, L.R. Biosynthesis, electrochemical, antimicrobial and antioxidant studies of silver nanoparticles mediated by Talinum triangulare aqueous leaf extract. J. Clust. Sci. 2017, 28, 309–330. [Google Scholar] [CrossRef]
- Pandey, K.; Sharma, P.K.; Dudhe, R. Antioxidant and anti-inflammatory activity of ethanolic extract of Parthenium hysterophorus Linn. Asian J. Pharm. Clin. Res. 2012, 5, 28–31. [Google Scholar]
- LOHUMI, P.; KUMAR, T.; NOGAI, L. EVALUATION OF ETHANOLIC ROOT EXTRACT OF PARTHENIUM HYSTEROPHORUS LINN FOR ANTIOXIDANT AND ANTI-INFLAMMATORY ACTIVITY. Int. J. Curr. Pharm. Res. 2017, 9, 194–197. [Google Scholar] [CrossRef]
- Rees, D.; Palmer, R.; Moncada, S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc. Natl. Acad. Sci. USA 1989, 86, 3375–3378. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Gattorno, G.; Diaz, D.; Rendon, L.; Hernandez-Segura, G. Metallic nanoparticles from spontaneous reduction of silver (I) in DMSO. Interaction between nitric oxide and silver nanoparticles. J. Phys. Chem. B 2002, 106, 2482–2487. [Google Scholar] [CrossRef]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Azeez, L.; Ajibade, S.E.; Ojo, S.A.; Gueguim-Kana, E.B. Biogenic synthesis of silver nanoparticles using a pod extract of Cola nitida: Antibacterial and antioxidant activities and application as a paint additive. J. Taibah Univ. Sci. 2016, 10, 551–562. [Google Scholar] [CrossRef] [Green Version]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Azeez, M.A.; Yekeen, T.A.; Akinboro, A. Evaluation of some biosynthesized silver nanoparticles for biomedical applications: Hydrogen peroxide scavenging, anticoagulant and thrombolytic activities. J. Clust. Sci. 2017, 28, 1379–1392. [Google Scholar] [CrossRef]
- Nia, P.M.; Meng, W.P.; Alias, Y. Hydrogen peroxide sensor: Uniformly decorated silver nanoparticles on polypyrrole for wide detection range. Appl. Surf. Sci. 2015, 357, 1565–1572. [Google Scholar] [CrossRef]
- Wang, L.; Ma, S.; Yang, B.; Cao, W.; Han, X. Morphology-controlled synthesis of Ag nanoparticle decorated poly (o-phenylenediamine) using microfluidics and its application for hydrogen peroxide detection. Chem. Eng. J. 2015, 268, 102–108. [Google Scholar] [CrossRef]
- Chang, H.-F.; Yang, L.-L. Radical-scavenging and rat liver mitochondria lipid peroxidative inhibitory effects of natural flavonoids from traditional medicinal herbs. J. Med. Plants Res. 2012, 6, 997–1006. [Google Scholar]
- Lin, Y.-L.; Juan, I.-M.; Chen, Y.-L.; Liang, Y.-C.; Lin, J.-K. Composition of polyphenols in fresh tea leaves and associations of their oxygen-radical-absorbing capacity with antiproliferative actions in fibroblast cells. J. Agric. Food Chem. 1996, 44, 1387–1394. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef]
- Lateef, A.; Folarin, B.I.; Oladejo, S.M.; Akinola, P.O.; Beukes, L.S.; Gueguim-Kana, E.B. Characterization, antimicrobial, antioxidant, and anticoagulant activities of silver nanoparticles synthesized from Petiveria alliacea L. leaf extract. Prep. Biochem. Biotechnol. 2018, 48, 646–652. [Google Scholar] [CrossRef]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and treatment of colorectal cancer by natural agents from mother nature. Curr. Colorectal Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomathi, A.; Rajarathinam, S.X.; Sadiq, A.M.; Rajeshkumar, S. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug Deliv. Sci. Technol. 2020, 55, 101376. [Google Scholar] [CrossRef]
- Priya, K.; Vijayakumar, M.; Janani, B. Chitosan-mediated synthesis of biogenic silver nanoparticles (AgNPs), nanoparticle characterisation and in vitro assessment of anticancer activity in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2020, 149, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Al-Awady, M.J.; Balakit, A.A.; Al-Musawi, S.; Alsultani, M.J.; Kamil, A.; Alabbasi, M. Investigation of Anti-MRSA and Anticancer Activity of Eco-Friendly Synthesized Silver Nanoparticles from Palm Dates Extract. Nano Biomed. Eng. 2019, 11, 157–169. [Google Scholar] [CrossRef]
- Lovatel, R.; Neves, R.; Oliveira, G.; Mauler, R.; Crespo, J.; Carli, L.; Giovanela, M. Disinfection of biologically treated industrial wastewater using montmorillonite/alginate/nanosilver hybrids. J. Water Process Eng. 2015, 7, 273–279. [Google Scholar] [CrossRef]
- Prabu, D.; Tharani, C.; Narayanan, N.; Maheswaran, A. Biomedical evaluation of polymeric hydrogel dermal patches as wound dressing. Int. J. Adv. Pharm. Res. 2011, 2, 569–575. [Google Scholar]
- Shrivastava, S.; Dash, D. In Agrifood nanotechnology: A tiny revolution in food and agriculture. J. Nano Res. 2009, 6, 1–14. [Google Scholar] [CrossRef]
- Negahdary, M.; Omidi, S.; Eghbali-Zarch, A.; Mousavi, S.A.; Mohseni, G.; Moradpour, Y.; Rahimi, G. Plant synthesis of silver nanoparticles using Matricaria chamomilla plant and evaluation of its antibacterial and antifungal effects. Biomed. Res. 2015, 26, 794–799. [Google Scholar]
- Brahmachari, G.; Sarkar, S.; Ghosh, R.; Barman, S.; Mandal, N.C.; Jash, S.K.; Banerjee, B.; Roy, R. Sunlight-induced rapid and efficient biogenic synthesis of silver nanoparticles using aqueous leaf extract of Ocimum sanctum Linn. with enhanced antibacterial activity. Org. Med. Chem. Lett. 2014, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Balakrishnan, S.; Sivaji, I.; Kandasamy, S.; Duraisamy, S.; Kumar, N.S.; Gurusubramanian, G. Biosynthesis of silver nanoparticles using Myristica fragrans seed (nutmeg) extract and its antibacterial activity against multidrug-resistant (MDR) Salmonella enterica serovar Typhi isolates. Environ. Sci. Pollut. Res. 2017, 24, 14758–14769. [Google Scholar] [CrossRef] [PubMed]
- Sakat, S.; Tupe, P.; Juvekar, A. Gastroprotective effect of methanol extract of Oxalis corniculata Linn (whole plant) experimental animals. Planta Medica 2010, 76, P090. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; De Tommasi, N.; Di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from bauhinia t arapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Siddiquee, M.A.; ud din Parray, M.; Mehdi, S.H.; Alzahrani, K.A.; Alshehri, A.A.; Malik, M.A.; Patel, R. Green synthesis of silver nanoparticles from Delonix regia leaf extracts: In-vitro cytotoxicity and interaction studies with bovine serum albumin. Mater. Chem. Phys. 2020, 242, 122493. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, A.; Farooq, M.A.; Ahsan Bajwa, A.; Parveen, A. Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation. Molecules 2020, 25, 3324. https://doi.org/10.3390/molecules25153324
Ahsan A, Farooq MA, Ahsan Bajwa A, Parveen A. Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation. Molecules. 2020; 25(15):3324. https://doi.org/10.3390/molecules25153324
Chicago/Turabian StyleAhsan, Anam, Muhammad Asim Farooq, Ali Ahsan Bajwa, and Amna Parveen. 2020. "Green Synthesis of Silver Nanoparticles Using Parthenium Hysterophorus: Optimization, Characterization and In Vitro Therapeutic Evaluation" Molecules 25, no. 15: 3324. https://doi.org/10.3390/molecules25153324