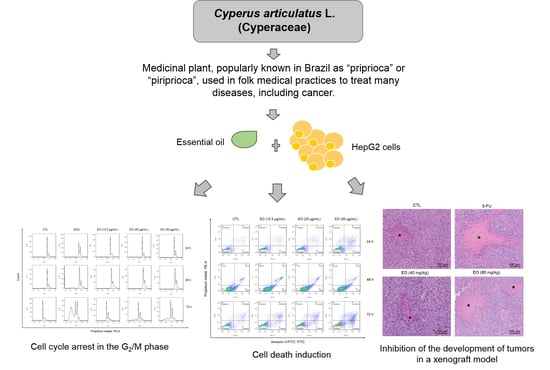
Cyperus articulatus L. (Cyperaceae) Rhizome Essential Oil Causes Cell Cycle Arrest in the G2/M Phase and Cell Death in HepG2 Cells and Inhibits the Development of Tumors in a Xenograft Model
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of Cyperus articulatus Rhizome Essential Oil
2.2. Cyperus articulatus Rhizome Essential Oil Induces Cytotoxicity in a Panel of Cancer Cell Lines
2.3. Cyperus articulatus Rhizome Essential Oil Causes Cell Cycle Arrest in the G2/M Phase and Cell Death in HepG2 Cells
2.4. Cyperus articulatus Rhizome Essential Oil Inhibits Tumor Development in a Xenograft Model
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Essential Oil Extraction
4.3. Chemical Analysis
4.4. In Vitro Cytotoxicity
4.4.1. Cells
4.4.2. Cytotoxicity Assay
4.4.3. Trypan Blue Exclusion Assay
4.4.4. Internucleosomal DNA Fragmentation and Cell Cycle Distribution
4.4.5. May-Grunwald-Giemsa Staining
4.4.6. Cell Death Quantification
4.5. In Vivo Antitumor Study
4.5.1. Animals
4.5.2. Human Hepatocellular Carcinoma Xenograft Model
4.5.3. Toxicological Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar] [PubMed]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, B.A. Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Oliveira, J.; Carreira, L.M.M.; Guilhon, G.M.S.P. Yield and chemical composition of the essential oil of the stems and rhizomes of Cyperus articulatus L. cultivated in the state of Pará, Brazil. J. Essent. Oil Res. 2006, 18, 10–12. [Google Scholar] [CrossRef]
- Taylor, L. Piri-piri. The Tropical Plant Database. Raintree. 2019. Available online: http://www.rain-tree.com/piri-piri.htm (accessed on 27 December 2019).
- Hoet, P. The use of certain plants from the traditional pharmacopoeia of Peru. Plant Med. Phytother. 1980, 14, 193–201. [Google Scholar]
- Feo, V. Medicinal and magical plants in the northern Peruvian andes. Fitoterapia 1992, 63, 417–440. [Google Scholar]
- Valera, G.A. Medicina Indigena. Las Plantas Medicinales y su Beneficio en la Salud (Shipibo—Conibo); Centro Orientamento Educativo: Pulcalpa, Peru, 1994. [Google Scholar]
- Bum, E.N.; Meier, C.L.; Urwyler, S.; Wang, Y.; Herrling, P.L. Extracts from rhizomes of Cyperus articulatus (Cyperaceae) displace [3H] CGP39653 and [3H] glycine binding from cortical membranes and selectively inhibit NMDA receptor-mediated neurotransmission. J. Ethnopharmacol. 1996, 54, 103–111. [Google Scholar] [CrossRef]
- Milliken, W.; Albert, B. The use of medicinal plants by the yanomami indians of Brazil. Econ. Bot. 1996, 50, 10–25. [Google Scholar] [CrossRef]
- Nguta, J.M.; Appiah-Opong, R.; Nyarko, A.K.; Yeboah-Manu, D.; Addo, P.G. Medicinal plants used to treat TB in Ghana. Int. J. Mycobacteriol. 2015, 4, 116–123. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Santiváñez-Acosta, R.; Pari-Olarte, B.; Enciso-Roca, E.; Montes, V.M.C.; Acevedo, J.L.A. Anticonvulsant effect of ethanolic extract of Cyperus articulatus L. leaves on pentylenetetrazol induced seizure in mice. J. Tradit. Complement. Med. 2017, 8, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Rakotonirina, V.S.; Bum, E.N.; Rakotonirina, A.; Bopelet, M. Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia 2001, 72, 22–29. [Google Scholar] [CrossRef]
- Duarte, M.C.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.; Delarmelina, C. Anti-Candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef]
- Rukunga, G.M.; Muregi, F.W.; Omar, S.A.; Gathirwa, J.W.; Muthaura, C.N.; Peter, M.G.; Heydenreich, M.; Mungai, G.M. Anti-plasmodial activity of the extracts and two sesquiterpenes from Cyperus articulatus. Fitoterapia 2008, 79, 188–190. [Google Scholar] [CrossRef]
- Metuge, J.A.; Nyongbela, K.D.; Mbah, J.A.; Samje, M.; Fotso, G.; Babiaka, S.B.; Cho-Ngwa, F. Anti-Onchocerca activity and phytochemical analysis of an essential oil from Cyperus articulatus L. BMC Complement. Altern. Med. 2014, 14, 223. [Google Scholar] [CrossRef] [Green Version]
- Freires, I.A.; Bueno-Silva, B.; Galvão, L.C.; Duarte, M.C.; Sartoratto, A.; Figueira, G.M.; de Alencar, S.M.; Rosalen, P.L. The effect of essential oils and bioactive fractions on Streptococcus mutans and Candida albicans biofilms: A confocal analysis. Evid. Based Complement. Alternat. Med. 2015, 2015, 871316. [Google Scholar] [CrossRef] [Green Version]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef]
- Ferraz, R.P.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.; Silva, T.B.; Machado, W.J.; Prata, A.P.; Costa, E.V.; Moraes, V.R.; Nogueira, P.C.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.C.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.; Dias, R.B.; Soares, M.B.; Prata, A.P.; Rocha, C.A.; Costa, E.V.; Bezerra, D.P. Antitumor properties of the essential oil from the leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar] [CrossRef]
- Silva, N.C.; Gonçalves, S.F.; Araújo, L.L.; Kasper, A.A.M.; Fonseca, A.L.; Sartoratto, A.; Castro, K.C.F.; Moraes, T.M.P.; Baratto, C.; Varotti, F.P.; et al. In vitro and in vivo antimalarial activity of the volatile oil of Cyperus articulatus (Cyperaceae). Acta Amaz. 2019, 49, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Hassanein, H.D.; Nazif, N.M.; Shahat, A.A.; Hammouda, F.M.; Aboutable, E.A.; Saleh, M.A. Chemical diversity of essential oils from Cyperus articulatus, Cyperus esculentus and Cyperus papyrus. J. Essent. Oil Bear. Plants 2014, 17, 251–264. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Ribeiro, S.S.; Jesus, A.M.; Anjos, C.S.; Silva, T.B.; Santos, A.D.; Jesus, J.R.; Andrade, M.S.; Sampaio, T.S.; Gomes, W.F.; Alves, P.B.; et al. Evaluation of the cytotoxic activity of some Brazilian medicinal plants. Planta Med. 2012, 78, 1601–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, T.B.; Costa, C.O.; Galvão, A.F.; Bomfim, L.M.; Rodrigues, A.C.; Mota, M.C.; Dantas, A.A.; Santos, T.R.; Soares, M.B.; Bezerra, D.P. Cytotoxic potential of selected medicinal plants in northeast Brazil. BMC Complement. Altern. Med. 2016, 16, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.C.B.C.; Oliveira, F.P.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Costa, E.V.; Silva, F.M.A.D.; Rocha, W.C.; Koolen, H.H.F.; et al. In vitro and in vivo anti-leukemia activity of the stem bark of Salacia impressifolia (Miers) A. C. Smith (Celastraceae). J. Ethnopharmacol. 2019, 231, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.G.A.; Anunciação, T.A.D.; Araujo, M.D.S.; Souza, C.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Silva, F.M.A.D.; Koolen, H.H.F.; et al. In vitro and in vivo growth inhibition of human acute promyelocytic leukemia HL-60 cells by Guatteria megalophylla Diels (Annonaceae) leaf essential oil. Biomed. Pharmacother. 2020, 122, 109713. [Google Scholar] [CrossRef]
- Kilani, S.; Ledauphin, J.; Bouhlel, I.; Ben Sghaier, M.; Boubaker, J.; Skandrani, I.; Mosrati, R.; Ghedira, K.; Barillier, D.; Chekir-Ghedira, L. Comparative study of Cyperus rotundus essential oil by a modified GC/MS analysis method. Evaluation of its antioxidant, cytotoxic, and apoptotic effects. Chem. Biodivers. 2008, 5, 729–742. [Google Scholar] [CrossRef]
- Hu, Q.P.; Cao, X.M.; Hao, D.L.; Zhang, L.L. Chemical composition, antioxidant, DNA damage protective, cytotoxic and antibacterial activities of Cyperus rotundus rhizomes essential oil against foodborne pathogens. Sci. Rep. 2017, 7, 45231. [Google Scholar] [CrossRef] [Green Version]
- Memariani, T.; Hosseini, T.; Kamali, H.; Mohammadi, A.; Ghorbani, M.; Shakeri, A.; Spandidos, D.A.; Tsatsakis, A.M.; Shahsavand, S. Evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the PC3 and MCF7 cancer cell lines. Oncol. Lett. 2016, 11, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.Q.; Xu, B.; Mao, J.W.; Wei, F.X.; Li, M.; Liu, T.; Jin, X.B.; Zhang, L.R. Inhibitory effects of α-pinene on hepatoma carcinoma cell proliferation. Asian Pac. J. Cancer Prev. 2014, 7, 3293–3297. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-Pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Togar, B.; Tatar, A.; Geyıkoglu, F.; Hacımuftuoglu, A. Cytotoxic and cytogenetic effects of α-copaene on rat neuron and N2a neuroblastoma cell lines. Biologia 2014, 69, 936–942. [Google Scholar] [CrossRef]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, C.; Qiu, J.; Wang, Z. Essential oil from Pinus Koraiensis pinecones inhibits gastric cancer cells via the HIPPO/YAP signaling pathway. Molecules 2019, 24, 3851. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Expression of P53, BAX, and BCL-2 in human malignant melanoma and squamous cell carcinoma cells after tea tree oil treatment in vitro. Cytotechnology 2018, 71, 461–473. [Google Scholar] [CrossRef] [PubMed]
- John Wiley & Sons Ltd. Wiley Registry 8th Edition/NIST 05 Mass Spectral Library, 8th ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]-thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Santos, L.S.; Silva, V.R.; Menezes, L.R.A.; Soares, M.B.P.; Costa, E.V.; Bezerra, D.P. Xylopine induces oxidative stress and causes G2/M phase arrest, triggering caspase-mediated apoptosis by p53-independent pathway in HCT116 cells. Oxid. Med. Cell Longev. 2017, 2017, 7126872. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.R.; Correa, R.S.; Santos, L.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. A ruthenium-based 5-fluorouracil complex with enhanced cytotoxicity and apoptosis induction action in HCT116 cells. Sci. Rep. 2018, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Lima, E.J.S.P.; Alves, R.G.; D Elia, G.M.A.; Anunciação, T.A.D.; Silva, V.R.; Santos, L.S.; Soares, M.B.P.; Cardozo, N.M.D.; Costa, E.V.; Silva, F.M.A.D.; et al. Antitumor effect of the essential oil from the leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules 2018, 23, 2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, N.C.; Neves, S.P.; Dias, R.B.; Valverde, L.F.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Dos Santos, E.R.; Oliveira, R.M.M.; Carlos, R.M.; et al. A novel ruthenium complex with xanthoxylin induces S-phase arrest and causes ERK1/2-mediated apoptosis in HepG2 cells through a p53-independent pathway. Cell Death Dis. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, S.P.; Carvalho, N.C.; Silva, M.M.; Rodrigues, A.C.B.C.; Bomfim, L.M.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Batista, A.A.; et al. Ruthenium complexes containing heterocyclic thioamidates trigger caspase-mediated apoptosis through MAPK signaling in human hepatocellular carcinoma cells. Front. Oncol. 2019, 9, 562. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the EO is not available from the authors. |









| Peak Number | Compound | Retention Time (min) | RI | ProportionalArea (%) |
|---|---|---|---|---|
| 1 | α-Pinene | 5.50 | 931 | 8.26 ± 0.74 |
| 2 | Verbenene | 6.05 | 967 | 0.44 ± 0.06 |
| 3 | β-Pinene | 6.73 | 975 | 4.54 ± 0.52 |
| 4 | p-Cymene | 8.31 | 1025 | 0.42 ± 0.05 |
| 5 | Limonene | 8.50 | 1028 | 0.93 ± 0.11 |
| 6 | Isopinocarveol | 13.1 | 1160 | 2.13 ± 0.20 |
| 7 | β-Phellandren-8-ol | 13.5 | 1163 | 0.32 ± 0.02 |
| 8 | α-Phellandren-8-ol | 14.4 | 1168 | 0.81 ± 0.10 |
| 9 | Terpinen-4-ol | 14.9 | 1174 | 0.22 ± 0.05 |
| 10 | α-Terpineol | 15.5 | 1190 | 0.63 ± 0.03 |
| 11 | Myrtenol | 15.8 | 1198 | 3.47 ± 0.37 |
| 12 | Verbenone | 16.4 | 1205 | 0.71 ± 0.08 |
| 13 | α-Copaene | 24.6 | 1375 | 4.83 ± 0.45 |
| 14 | β-Elemene | 25.5 | 1394 | 0.35 ± 0.02 |
| 15 | α-Gurjunene | 25.8 | 1409 | 1.55 ± 0.17 |
| 16 | β-Caryophyllene | 26.1 | 1435 | 1.03 ± 0.11 |
| 17 | β-Copaene | 27.8 | 1440 | 1.22 ± 0.12 |
| 18 | Unknown | 28.9 | - | 0.84 ± 0.05 |
| 19 | Unknown | 29.9 | - | 0.15 ± 0.01 |
| 20 | Caryophyllene oxide | 30.7 | 1550 | 4.82 ± 0.44 |
| 21 | Unknown | 31.4 | - | 1.51 ± 0.11 |
| 22 | β-Copaen-4-α-ol | 32.3 | 1570 | 4.74 ± 0.40 |
| 23 | Unknown | 37.7 | - | 0.72 ± 0.04 |
| 24 | Unknown | 38.3 | - | 1.82 ± 0.15 |
| 25 | Spathulenol | 39.0 | 1588 | 3.68 ± 0.38 |
| 26 | Unknown | 43.8 | - | 0.43 ± 0.05 |
| 27 | Globulol | 44.9 | 1591 | 2.72 ± 0.29 |
| 28 | Unknown | 45.1 | - | 0.90 ± 0.08 |
| 29 | Unknown | 45.5 | - | 0.58 ± 0.06 |
| 30 | Muskatone | 46.0 | 1681 | 11.60 ± 1.19 |
| 31 | Cyperol | 46.5 | 1684 | 1.84 ± 0.15 |
| 32 | Unknown | 46.9 | - | 0.92 ± 0.09 |
| 33 | Pogostol | 47.2 | 1687 | 6.36 ± 0.88 |
| 34 | Unknown | 47.4 | - | 1.60 ± 0.21 |
| 35 | Unknown | 47.7 | - | 1.02 ± 0.10 |
| 36 | Unknown | 48.0 | - | 1.30 ± 0.16 |
| 37 | (E,E)-Farnesol | 48.1 | 1740 | 1.43 ± 0.15 |
| 38 | Cyclocolorenone | 48.4 | 1753 | 10.30 ± 1.02 |
| 39 | Unknown | 49.3 | - | 0.21 ± 0.02 |
| 40 | Unknown | 49.5 | - | 0.49 ± 0.05 |
| 41 | Unknown | 50.2 | - | 0.19 ± 0.01 |
| 42 | (E)-Isogeraniol | 51.6 | 1817 | 0.72 ± 0.04 |
| Σhydrocarbon monoterpenes | 14.59 | |||
| Σoxygenated monoterpenes | 8.29 | |||
| Σhydrocarbon sesquiterpenes | 8.98 | |||
| Σoxygenated sesquiterpenes | 47.49 | |||
| Σtotal identified | 80.07 |
| Cell Lines | Histological Type | IC50 (95% CI) in µg/mL | ||
|---|---|---|---|---|
| EO | DOX | 5-FU | ||
| Cancer cells | ||||
| HepG2 | Human hepatocellular carcinoma | 28.5 (23.8–36.4) | 0.03 (0.01–0.2) | 0.2 (0.1–0.4) |
| HCT116 | Human colon carcinoma | >50 | 0.1 (0.1–0.2) | 0.5 (0.3–1.1) |
| MCF-7 | Human breast adenocarcinoma | 36.7 (26.7–50.5) | 0.3 (0.2–0.4) | 1.8 (0.7–2.3) |
| HL-60 | Human promyelocytic leukemia | 33.51 (27.3–41.2) | 0.04 (0.02–0.08) | 1.6 (1.2–2.2) |
| B16-F10 | Mouse melanoma | 39.7 (32.1–49.0) | 0.2 (0.2–0.2) | 0.5 (0.3–0.8) |
| Non-cancerous cell | ||||
| MRC-5 | Human lung fibroblast | 46.0 (39.6–53.6) | 0.2 (0.1–0.5) | 7.5 (5.2–11.0) |
| Cancer Cells | Non-Cancerous Cells | ||
|---|---|---|---|
| MRC-5 | |||
| EO | DOX | 5-FU | |
| HepG2 | 1.6 | 69.7 | 37.5 |
| HCT116 | n.d. | 16.1 | 15 |
| MCF-7 | 1.3 | 7.5 | 4.2 |
| HL-60 | 1.4 | 52.3 | 4.7 |
| B16-F10 | 1.2 | 11.0 | 15 |
| Parameters | CTL | 5-FU | EO | |
|---|---|---|---|---|
| Dose (mg/kg) | - | 10 | 40 | 80 |
| Survival | 20/20 | 10/10 | 10/10 | 10/10 |
| Initial body weight (g) | 21.4 ± 0.5 | 19.6 ± 0.6 | 21.0 ± 0.4 | 21.0 ± 0.5 |
| Final body weight (g) | 22.1 ± 0.5 | 20.5 ± 0.5 | 19.8 ± 0.9 | 21.9 ± 0.5 |
| Liver (g/100 g body weight) | 4.8 ± 0.2 | 4.8 ± 0.2 | 5.4 ± 0.3 | 4.9 ± 0.3 |
| Kidney (g/100 g body weight) | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.1 |
| Heart (g/100 g body weight) | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Lung (g/100 g body weight) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| Parameters | CTL | 5-FU | EO | |
|---|---|---|---|---|
| Dose (mg/kg) | - | 10 | 40 | 80 |
| Erythrocytes (106/mm3) | 5.2 ± 1.1 | 7.6 ± 0.8 | 5.4 ± 1.1 | 6.7 ± 1.3 |
| Hemoglobin (g/dL) | 21.2 ± 4.8 | 17.7 ± 3.1 | 26.8 ± 0.7 | 18.0 ± 1.4 |
| MCV (fL) | 43.8 ± 0.4 | 45.0 ± 3.0 | 41.5 ± 0.5 | 44.8 ± 0.5 |
| Platelets (103/mm3) | 247.2 ± 38.5 | 222.1 ± 41.6 | 456.7 ± 112.2 | 464.1 ± 62.4 |
| Leukocytes (103/mm3) | 5.2 ± 0.8 | 2.5 ± 0.6 | 7.6 ± 0.7 | 2.9 ± 0.7 |
| Differential leukocytes (%) | ||||
| Granulocytes | 24.1 | 28.4 | 25.7 | 34.3 |
| Lymphocytes | 41.5 | 46.1 | 52.2 | 40.4 |
| Monocytes | 33.6 | 25.5 | 21.2 | 25.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, M.L.; Lima, E.J.S.P.d.; Adrião, A.A.X.; Fontes, S.S.; Silva, V.R.; Santos, L.d.S.; Soares, M.B.P.; Dias, R.B.; Rocha, C.A.G.; Costa, E.V.; et al. Cyperus articulatus L. (Cyperaceae) Rhizome Essential Oil Causes Cell Cycle Arrest in the G2/M Phase and Cell Death in HepG2 Cells and Inhibits the Development of Tumors in a Xenograft Model. Molecules 2020, 25, 2687. https://doi.org/10.3390/molecules25112687
Nogueira ML, Lima EJSPd, Adrião AAX, Fontes SS, Silva VR, Santos LdS, Soares MBP, Dias RB, Rocha CAG, Costa EV, et al. Cyperus articulatus L. (Cyperaceae) Rhizome Essential Oil Causes Cell Cycle Arrest in the G2/M Phase and Cell Death in HepG2 Cells and Inhibits the Development of Tumors in a Xenograft Model. Molecules. 2020; 25(11):2687. https://doi.org/10.3390/molecules25112687
Chicago/Turabian StyleNogueira, Mateus L., Emilly J. S. P. de Lima, Asenate A. X. Adrião, Sheila S. Fontes, Valdenizia R. Silva, Luciano de S. Santos, Milena B. P. Soares, Rosane B. Dias, Clarissa A. Gurgel Rocha, Emmanoel V. Costa, and et al. 2020. "Cyperus articulatus L. (Cyperaceae) Rhizome Essential Oil Causes Cell Cycle Arrest in the G2/M Phase and Cell Death in HepG2 Cells and Inhibits the Development of Tumors in a Xenograft Model" Molecules 25, no. 11: 2687. https://doi.org/10.3390/molecules25112687