Effect of Angelica gigas Nakai Ethanol Extract and Decursin on Human Pancreatic Cancer Cells
Abstract
:1. Introduction
2. Results
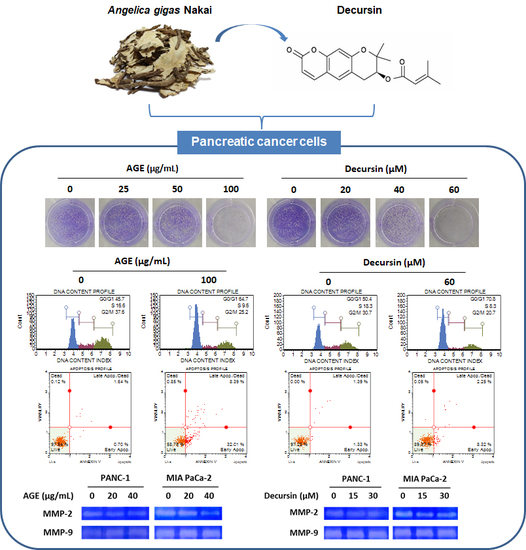
2.1. AGE and Decursin Inhibited Proliferation of PANC-1 and MIA PaCa-2 Cells
2.2. AGE and Decursin Induced Cell Cycle Arrest of PC Cells by Decreasing Cyclin–CDK Expression
2.3. AGE and Decursin Induced Apoptosis of PC Cells
2.4. AGE and Decursin Decreased MMP-2 and MMP-9 Expression in PC Cells by Regulating p38 Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of AGE
4.3. Ultra-Performance Liquid Chromatography
4.4. Cell Lines and Cell Culture
4.5. Measurement of Cell Viability
4.6. Colony Formation
4.7. Flow Cytometry Analysis for Cell Cycle Arrest
4.8. Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Assay
4.9. Annexin V Assay
4.10. Western Blotting
4.11. RNA Extraction and Real-Time RT-PCR
4.12. Detection of MMP-2 and MMP-9 Activity
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- King, K.L.; Cidlowski, J.A. Cell cycle and apoptosis: Common pathways to life and death. J. Cell. Biochem. 1995, 58, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.; Jacks, T.; Pavletich, N.P. The cell cycle and cancer. Proc. Natl. Acad. Sci. USA 1997, 94, 2776–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnum, K.J.; O’Connell, M.J. Cell cycle regulation by checkpoints. Methods Mol. Biol. 2014, 1170, 29–40. [Google Scholar] [PubMed] [Green Version]
- Malumbres, M.; Barbacid, M. Mammalian cyclin-dependent kinases. Trends Biochem. Sci. 2005, 30, 630–641. [Google Scholar] [CrossRef]
- Said, A.H.; Raufman, J.P.; Xie, G. The role of matrix metalloproteinases in colorectal cancer. Cancers 2014, 6, 366–375. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Shin, J.I.; Lee, S.; Lee, Y.G.; Kim, J.B.; Kwon, H.C.; Kim, S.H.; Kim, I.; Lee, K.; Han, Y.S. Angelica gigas Nakai Has Synergetic Effects on Doxorubicin-Induced Apoptosis. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Oh, S.R.; Ok, S.; Jung, T.S.; Jeon, S.O.; Park, J.M.; Jung, J.W.; Ryu, D.S. Protective effect of decursin and decursinol angelate-rich Angelica gigas Nakai extract on dextran sulfate sodium-induced murine ulcerative colitis. Asian Pac. J. Trop. Med. 2017, 10, 864–870. [Google Scholar] [CrossRef]
- Kim, K.J.; Yeon, J.T.; Choi, S.W.; Moon, S.H.; Ryu, B.J.; Yu, R.; Park, S.J.; Kim, S.H.; Son, Y.J. Decursin inhibits osteoclastogenesis by downregulating NFATc1 and blocking fusion of pre-osteoclasts. Bone 2015, 81, 208–216. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.J.; Lee, E.O.; Lee, J.H.; Lee, K.S.; Kim, K.H.; Kim, S.H.; Lu, J. In vivo anti-cancer activity of Korean Angelica gigas and its major pyranocoumarin decursin. Am. J. Chin. Med. 2009, 37, 127–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, J.Y.; Han, Y.H.; Mun, J.G.; Park, S.H.; Jeon, H.D.; Hong, S.H. Gomisin A Suppresses Colorectal Lung Metastasis by Inducing AMPK/p38-Mediated Apoptosis and Decreasing Metastatic Abilities of Colorectal Cancer Cells. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.H.; Park, K.K.; Park, S.K.; Kim, Y.C.; Kim, Y.S.; Lee, S.K.; Chung, W.Y. Decursin and decursinol from Angelica gigas inhibit the lung metastasis of murine colon carcinoma. Phytother. Res. 2011, 25, 959–964. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.H.; Kim, E.J.; Kim, W.J.; Suk, K.; Kim, J.H.; Song, G.Y.; Lee, W.H. A novel derivative of decursin, CSL-32, blocks migration and production of inflammatory mediators and modulates PI3K and NF-kappaB activities in HT1080 cells. Cell Biol. Int. 2012, 36, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Noh, E.M.; Kim, M.S.; Hwang, J.K.; Hwang, H.Y.; Ryu, D.G.; Kim, H.J.; Yu, H.N.; You, Y.O.; Kim, J.S.; et al. Decursin prevents TPA-induced invasion through suppression of PKCalpha/p38/NF-kappaB-dependent MMP-9 expression in MCF-7 human breast carcinoma cells. Int. J. Oncol. 2014, 44, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Moon, J.S.; Lee, S.; Yim, D.; Singh, R.P. Inhibition of angiogenic attributes by decursin in endothelial cells and ex vivo rat aortic ring angiogenesis model. Indian J. Exp. Biol. 2011, 49, 848–856. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Singh, D.; Upadhyay, G.; Srivastava, R.K.; Shankar, S. Recent advances in pancreatic cancer: Biology, treatment, and prevention. Biochim. Biophys. Acta 2015, 1856, 13–27. [Google Scholar] [CrossRef]
- Kim, B.S.; Seo, H.; Kim, H.J.; Bae, S.M.; Son, H.N.; Lee, Y.J.; Ryu, S.; Park, R.W.; Nam, J.O. Decursin from Angelica gigas Nakai Inhibits B16F10 Melanoma Growth Through Induction of Apoptosis. J. Med. Food 2015, 18, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.R.; Lee, J.H.; Kim, J.Y.; Park, K.W.; Jeong, I.Y.; Shim, K.H.; Lee, M.K.; Seo, K.I. Decursin from Angelica gigas Nakai induces apoptosis in RC-58T/h/SA#4 primary human prostate cancer cells via a mitochondria-related caspase pathway. Food Chem. Toxicol. 2011, 49, 2517–2523. [Google Scholar]
- Kim, W.J.; Lee, S.J.; Choi, Y.D.; Moon, S.K. Decursin inhibits growth of human bladder and colon cancer cells via apoptosis, G1-phase cell cycle arrest and extracellular signal-regulated kinase activation. Int. J. Mol. Med. 2010, 25, 635–641. [Google Scholar]
- Kim, E.; Nam, J.; Chang, W.; Zulfugarov, I.S.; Okhlopkova, Z.M.; Olennikov, D.; Chirikova, N.K.; Kim, S.W. Angelica gigas Nakai and Decursin Downregulate Myc Expression to Promote Cell Death in B-cell Lymphoma. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizzotto, M.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenols of selected peach and plum genotypes reduce cell viability and inhibit proliferation of breast cancer cells while not affecting normal cells. Food Chem. 2014, 164, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Daskiewicz, J.B.; Depeint, F.; Viornery, L.; Bayet, C.; Comte-Sarrazin, G.; Comte, G.; Gee, J.M.; Johnson, I.T.; Ndjoko, K.; Hostettmann, K.; et al. Effects of flavonoids on cell proliferation and caspase activation in a human colonic cell line HT29: An SAR study. J. Med. Chem. 2005, 48, 2790–2804. [Google Scholar] [CrossRef]
- Nichenametla, S.N.; Taruscio, T.G.; Barney, D.L.; Exon, J.H. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 2006, 46, 161–183. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [Green Version]
- Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Buchler, M.; et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 2000, 85, 14–20. [Google Scholar] [CrossRef]
- Knapinska, A.M.; Estrada, C.A.; Fields, G.B. The Roles of Matrix Metalloproteinases in Pancreatic Cancer. Prog. Mol. Biol. Transl. Sci. 2017, 148, 339–354. [Google Scholar] [PubMed]
- Pagliara, V.; Nasso, R.; Di Donato, P.; Finore, I.; Poli, A.; Masullo, M.; Arcone, R. Lemon Peel Polyphenol Extract Reduces Interleukin-6-Induced Cell Migration, Invasiveness, and Matrix Metalloproteinase-9/2 Expression in Human Gastric Adenocarcinoma MKN-28 and AGS Cell Lines. Biomolecules 2019, 9, 833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; He, T.; Zhao, K.; Xing, C. Anti-metastatic activity of fangchinoline in human gastric cancer AGS cells. Oncol. Lett. 2017, 13, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arcone, R.; Palma, M.; Pagliara, V.; Graziani, G.; Masullo, M.; Nardone, G. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-alpha induced Matrix Metalloproteinase-9/2. Biochim. Open 2016, 3, 56–63. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Z.; Chen, M.; Li, C.; Liu, L.; Li, Y. Silibinin inhibits the migration and invasion of human gastric cancer SGC7901 cells by downregulating MMP-2 and MMP-9 expression via the p38MAPK signaling pathway. Oncol. Lett. 2017, 14, 7577–7582. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, P.; Ye, J.; Liao, Y.; Du, Z.; Chen, F.; Juanjuan, H.; Zhang, S.; Zhai, W. Oxymatrine inhibits the migration and invasion of hepatocellular carcinoma cells by reducing the activity of MMP-2/-9 via regulating p38 signaling pathway. J. Cancer 2019, 10, 5397–5403. [Google Scholar] [CrossRef]
- Kupiec, T.P. Quality-control analytical methods: High-performance liquid chromatography. Int. J. Pharm. Compd. 2004, 8, 223–227. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |





| Parameters | Condition | ||
|---|---|---|---|
| Instrument | 1290 Infinity UPLC System | ||
| Detector | DAD | ||
| Column | RP-Amide (2.1 × 150 mm, 2 μm) | ||
| Mobile phase | A: 100% acetonitrile (v/v, %) B: 0.1% phosphoric acid in water (v/v, %) | ||
| Gradient condition | Time (min) | A (%) | B (%) |
| 0 | 31 | 69 | |
| 35 | 31 | 69 | |
| 36 | 100 | 0 | |
| 40 | 100 | 0 | |
| Injection volume (μL) | 0.1 | ||
| Flow rate (mL/min) | 0.4 | ||
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Cyclin D1 | ATGCCAACCTCCTCAACGAC | GGCTCTTTTTCACGGGCTCC |
| CDK4 | GTGCAGTCGGTGGTACCTG | TTCGCTTGTGTGGGTTAAAA |
| GAPDH | CTGCACCACCAACTGCTTAG | TTCAGCTCAGGGATGACCTT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kweon, B.; Han, Y.-H.; Kee, J.-Y.; Mun, J.-G.; Jeon, H.D.; Yoon, D.H.; Choi, B.-M.; Hong, S.-H. Effect of Angelica gigas Nakai Ethanol Extract and Decursin on Human Pancreatic Cancer Cells. Molecules 2020, 25, 2028. https://doi.org/10.3390/molecules25092028
Kweon B, Han Y-H, Kee J-Y, Mun J-G, Jeon HD, Yoon DH, Choi B-M, Hong S-H. Effect of Angelica gigas Nakai Ethanol Extract and Decursin on Human Pancreatic Cancer Cells. Molecules. 2020; 25(9):2028. https://doi.org/10.3390/molecules25092028
Chicago/Turabian StyleKweon, Bitna, Yo-Han Han, Ji-Ye Kee, Jeong-Geon Mun, Hee Dong Jeon, Dae Hwan Yoon, Byung-Min Choi, and Seung-Heon Hong. 2020. "Effect of Angelica gigas Nakai Ethanol Extract and Decursin on Human Pancreatic Cancer Cells" Molecules 25, no. 9: 2028. https://doi.org/10.3390/molecules25092028