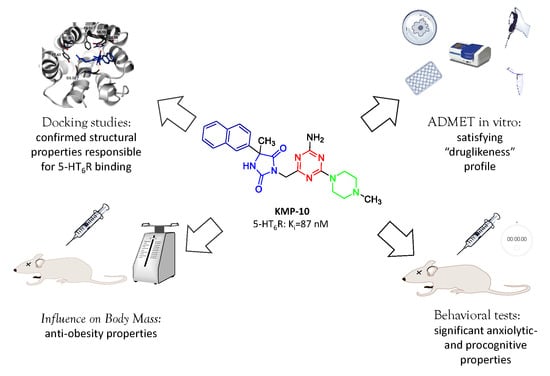
Are the Hydantoin-1,3,5-triazine 5-HT6R Ligands a Hope to a Find New Procognitive and Anti-Obesity Drug? Considerations Based on Primary In Vivo Assays and ADME-Tox Profile In Vitro
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Modeling
2.2. ADME-Tox Parameters In vitro
2.2.1. Permeability Assays
Parallel Artificial Membrane Permeability Assay (PAMPA)
Permeability Assay with Using Caco-2 Cells
2.2.2. Affinity to P-Glycoprotein
2.2.3. Plasma Protein Binding
2.2.4. Metabolic Stability
2.2.5. Drug–Drug Interactions (DDIs)
2.2.6. Ames Test
2.2.7. Hepatotoxicity Assay
2.2.8. “Druglikeness” of KMP-10 in Comparison to SUVN-502
2.3. Behavioral Test In vivo
2.3.1. Anxiolytic-Like Activity of Compound KMP-10
2.3.2. Novel Object Recognition (NOR) Test
2.4. Metabolic Test In vivo
2.4.1. Influence of KMP-10 on Caloric and Water Intakes of Obese Rats
2.4.2. Effect of KMP-10 on Calorie and Water Intakes in Rats Fed Standard Diet
2.4.3. Influence on the Amount of Peritoneal Adipose Tissue and Liver Mass
2.4.4. Influence of Diet or of KMP-10 on Blood Glucose, Cholesterol and Triglyceride Levels in the Model of Excessive Eating
2.4.5. The Influence of KMP-10 on Spontaneous Activity Test
2.4.6. Discussion on the Influence of KMP-10 on Metabolism
3. Materials and Methods
3.1. Molecular Modeling
3.2. ADME-Tox Parameters In vitro
3.2.1. References
3.2.2. Permeability Assay
3.2.3. Permeability Assay with Using Caco-2
- dc/dt—the change in concentration in the receiving compartment over time
- V—volume of the solution in the receiving compartment (mL)
- A—surface area of the membrane (cm2)
- C0—the initial concentration in the donor compartment (µM).
3.2.4. Affinity to P-glycoprotein
3.2.5. Plasma Protein Binding
- [A]—free concentration of drug
- [P]—free concentration of protein
- [AP]—concentration of drug A bound to the protein P.
3.2.6. Metabolic Stability
3.2.7. Drug–Drug Interaction (DDI)
3.2.8. Ames Test
3.2.9. Hepatotoxicity Assay
3.3. Behavioral Test In vivo
3.3.1. Animals
3.3.2. Drugs
3.3.3. Behavioral Procedures in Rats
Elevated Plus-Maze Test (EPM Test)
Exploratory Activity Measured in the EPM Test
Novel Object Recognition (NOR) Test
3.3.4. Statistical Analysis
3.4. Assays of Influence on Metabolism In vivo
3.4.1. Animals
3.4.2. Drugs
3.4.3. The Effect of KMP-10 on Food and Water Intake by Non-Obese Rats Fed Palatable Diet (Model of Excessive Eating)
3.4.4. The Effect of KMP-10 on Food and Water Intake by Non-Obese Rats Fed Only with Standard Diet
3.4.5. Influence of KMP-10 on Glucose, Cholesterol or Triglyceride Levels in Blood
3.4.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Monsma, F.J.; Shen, Y.; Ward, R.P.; Hamblin, M.W.; Sibley, D.R. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 1993, 43, 320–327. [Google Scholar] [PubMed]
- Ruat, M.; Traiffort, E.; Arrang, J.M.; Tardivellacombe, J.; Diaz, J.; Leurs, R.; Schwartz, J.C. A Novel Rat Serotonin (5-HT6) Receptor: Molecular Cloning, Localization and Stimulation of cAMP Accumulation. Biochem. Biophys. Res. Commun. 1993, 193, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.-M.; Rhim, H. The Serotonin-6 Receptor as a Novel Therapeutic Target. Exp. Neurobiol. 2011, 20, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karila, D.; Freret, T.; Bouet, V.; Boulouard, M.; Dallemagne, P.; Rochais, C. Therapeutic Potential of 5-HT 6 Receptor Agonists. J. Med. Chem. 2015, 58, 7901–7912. [Google Scholar] [CrossRef] [PubMed]
- Leiser, S.C.; Li, Y.; Pehrson, A.L.; Dale, E.; Smagin, G.; Sanchez, C. Serotonergic Regulation of Prefrontal Cortical Circuitries Involved in Cognitive Processing: A Review of Individual 5-HT Receptor Mechanisms and Concerted Effects of 5-HT Receptors Exemplified by the Multimodal Antidepressant Vortioxetine. ACS Chem. Neurosci. 2015, 6, 970–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforuk, A. The procognitive effects of 5-HT6 receptor ligands in animal models of schizophrenia. Rev. Neurosci. 2014, 25, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Benhamú, B.; Martín-Fontecha, M.; Vázquez-Villa, H.; Pardo, L.; López-Rodríguez, M.L. Serotonin 5-HT 6 Receptor Antagonists for the Treatment of Cognitive Deficiency in Alzheimer’s Disease. J. Med. Chem. 2014, 57, 7160–7181. [Google Scholar] [CrossRef]
- de Jong, I.E.M.; Mørk, A. Antagonism of the 5-HT6 receptor—Preclinical rationale for the treatment of Alzheimer’s disease. Neuropharmacology 2017, 125, 50–63. [Google Scholar] [CrossRef]
- Kenakin, T. Biased agonism. F1000 Biol. Rep. 2009, 1, 87. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Majouga, A.G.; Veselov, M.S.; Chufarova, N.V.; Baranovsky, S.S.; Filkov, G. Computational approaches to the design of novel 5-HT6R ligands. Rev. Neurosci. 2014, 25, 451–467. [Google Scholar] [CrossRef]
- Grychowska, K.; Satała, G.; Kos, T.; Partyka, A.; Colacino, E.; Chaumont-Dubel, S.; Bantreil, X.; Wesołowska, A.; Pawłowski, M.; Martinez, J.; et al. Novel 1H-Pyrrolo[3,2-c]quinoline Based 5-HT6 Receptor Antagonists with Potential Application for the Treatment of Cognitive Disorders Associated with Alzheimer’s Disease. ACS Chem. Neurosci. 2016, 7, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Marciniec, K.; Satała, G.; Canale, V.; Kos, T.; Partyka, A.; Jastrzębska-Więsek, M.; Wesołowska, A.; Basińska-Ziobroń, A.; Wójcikowski, J.; et al. N1-Azinylsulfonyl-1H-indoles: 5-HT6 Receptor Antagonists with Procognitive and Antidepressant-Like Properties. ACS Med. Chem. Lett. 2016, 7, 618–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelemen, Á.; Satała, G.; Bojarski, A.; Keserű, G. Spiro[pyrrolidine-3,3′-oxindoles] and Their Indoline Analogues as New 5-HT6 Receptor Chemotypes. Molecules 2017, 22, 2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staroń, J.; Mordalski, S.; Warszycki, D.; Satała, G.; Hogendorf, A.; Bojarski, A.J. Pyrano[2,3,4-cd]indole as a Scaffold for Selective Nonbasic 5-HT6R Ligands. ACS Med. Chem. Lett. 2017, 8, 390–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirogi, R.; Abraham, R.; Benade, V.; Medapati, R.B.; Jayarajan, P.; Bhyrapuneni, G.; Muddana, N.; Mekala, V.R.; Subramanian, R.; Shinde, A.; et al. SUVN-502, a novel, potent, pure, and orally active 5-HT6 receptor antagonist: Pharmacological, behavioral, and neurochemical characterization. Behav. Pharmacol. 2019, 30, 16–35. [Google Scholar] [CrossRef]
- Liu, K.G.; Robichaud, A.J. 5-HT6 antagonists as potential treatment for cognitive dysfunction. Drug Dev. Res. 2009, 70, 145–168. [Google Scholar] [CrossRef]
- Łażewska, D.; Kurczab, R.; Więcek, M.; Kamińska, K.; Satała, G.; Jastrzębska-Więsek, M.; Partyka, A.; Bojarski, A.J.; Wesołowska, A.; Kieć-Kononowicz, K.; et al. The computer-aided discovery of novel family of the 5-HT6 serotonin receptor ligands among derivatives of 4-benzyl-1,3,5-triazine. Eur. J. Med. Chem. 2017, 135, 117–124. [Google Scholar] [CrossRef]
- Łażewska, D.; Kurczab, R.; Więcek, M.; Satała, G.; Kieć-Kononowicz, K.; Handzlik, J. Synthesis and computer-aided analysis of the role of linker for novel ligands of the 5-HT6 serotonin receptor among substituted 1,3,5-triazinylpiperazines. Bioorg. Chem. 2019, 84, 319–325. [Google Scholar] [CrossRef]
- Kurczab, R.; Ali, W.; Łażewska, D.; Kotańska, M.; Jastrzębska, M.; Satała, G.; Więcek, M.; Lubelska, A.; Wesołowska, A.; Latacz, G.; et al. Computer-Aided Studies for Novel Arylhydantoin 1,3,5-Triazine Derivatives as 5-HT6 Serotonin Receptor Ligands with Antidepressive-Like, Anxiolytic and Antiobesity Action In vivo. Molecules 2018, 23, 2529. [Google Scholar] [CrossRef] [Green Version]
- Hogendorf, A.S.; Hogendorf, A.; Kurczab, R.; Kalinowska-Tłuścik, J.; Popik, P.; Nikiforuk, A.; Krawczyk, M.; Satała, G.; Lenda, T.; Knutelska, J.; et al. 2-Aminoimidazole-based antagonists of the 5-HT6 receptor—A new concept in aminergic GPCR ligand design. Eur. J. Med. Chem. 2019, 179, 1–15. [Google Scholar] [CrossRef]
- Chen, X.; Murawski, A.; Patel, K.; Crespi, C.L.; Balimane, P.V. A novel design of artificial membrane for improving the PAMPA model. Pharm. Res. 2008, 25, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Marć, M.A.; Satała, G.; Wilczyńska, D.; Kotańska, M.; Więcek, M.; Kamińska, K.; et al. The 1,3,5-Triazine Derivatives as Innovative Chemical Family of 5-HT6 Serotonin Receptor Agents with Therapeutic Perspectives for Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerns, E.; Di, L. Drug-Like Properties: Concept, Structure Design and Methods, From ADME to Toxicity Optimization; Academic press: Cambridge, MA, USA, 2008. [Google Scholar]
- Smetanova, L.; Stetinova, V.; Kholova, D.; Kvetina, J.; Smetana, J.; Svoboda, Z. Caco-2 cells and Biopharmaceutics Classification System (BCS) for prediction of transepithelial transport of xenobiotics (model drug: Caffeine). Neuro Endocrinol. Lett. 2009, 30, 101–105. [Google Scholar] [PubMed]
- Mullokandov, E.; Ahn, J.; Szalkiewicz, A. Protein Binding Drug-Drug Interaction between Warfarin and Tizoxanide in Human Plasma. Austin J. Pharmacol. Ther. 2014, 2, 7–9. [Google Scholar]
- Rosengren, A.M.; Karlsson, B.C.G.; Nicholls, I.A. Monitoring the distribution of warfarin in blood plasma. ACS Med. Chem. Lett. 2012, 3, 650–652. [Google Scholar] [CrossRef] [Green Version]
- Popiołek-Barczyk, K.; Łażewska, D.; Latacz, G.; Olejarz, A.; Makuch, W.; Stark, H.; Kieć-Kononowicz, K.; Mika, J. Antinociceptive effects of novel histamine H3 and H4 receptor antagonists and their influence on morphine analgesia of neuropathic pain in the mouse. Br. J. Pharmacol. 2018, 175, 2897–2910. [Google Scholar] [CrossRef] [Green Version]
- Nassar, A.F. Drug Metabolism Handbook: Concepts and Applications; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470439258. [Google Scholar]
- Nirogi, R.; Shinde, A.; Kambhampati, R.S.; Mohammed, A.R.; Saraf, S.K.; Badange, R.K.; Bandyala, T.R.; Bhatta, V.; Bojja, K.; Reballi, V.; et al. Discovery and Development of 1-[(2-Bromophenyl)sulfonyl]-5-methoxy-3-[(4-methyl-1-piperazinyl)methyl]-1H-indole Dimesylate Monohydrate (SUVN-502): A Novel, Potent, Selective and Orally Active Serotonin 6 (5-HT6) Receptor Antagonist for Potential Treatment of Alzheimer’s Disease. J. Med. Chem. 2017, 60, 1843–1859. [Google Scholar]
- Heal, D.J.; Smith, S.L.; Fisas, A.; Codony, X.; Buschmann, H. Selective 5-HT6 receptor ligands: Progress in the development of a novel pharmacological approach to the treatment of obesity and related metabolic disorders. Pharmacol. Ther. 2008, 117, 207–231. [Google Scholar] [CrossRef]
- Sargent, B.J.; Henderson, A.J. Targeting 5-HT receptors for the treatment of obesity. Curr. Opin. Pharmacol. 2011, 1, 52–58. [Google Scholar] [CrossRef]
- Vickers, S.P.; Jackson, H.C.; Cheetham, S.C. The utility of animal models to evaluate novel anti-obesity agents. Br. J. Pharmacol. 2011, 164, 1248–1262. [Google Scholar] [CrossRef] [Green Version]
- Kotańska, M.; Lustyk, K.; Bucki, A.; Marcinkowska, M.; Śniecikowska, J.; Kołaczkowski, M. Idalopirdine, a selective 5-HT6 receptor antagonist, reduces food intake and body weight in a model of excessive eating. Metab Brain Dis. 2018, 33, 733–740. [Google Scholar]
- Vaidyanathan, J.B.; Walle, T. Transport and metabolism of the tea flavonoid (−)-epicatechin by the human intestinal cell line Caco-2. Pharm. Res. 2001, 18, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Latacz, G.; Hogendorf, A.S.; Hogendorf, A.; Lubelska, A.; Wierońska, J.M.; Woźniak, M.; Cieślik, P.; Kieć-Kononowicz, K.; Handzlik, J.; Bojarski, A.J. Search for a 5-CT alternative. In vitro and in vivo evaluation of novel pharmacological tools: 3-(1-alkyl-1H-imidazol-5-yl)-1H-indole-5-carboxamides, low-basicity 5-HT7 receptor agonists. Medchemcomm 2018, 9, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Kucwaj-Brysz, K.; Wesołowska, A.; Kieć-Kononowicz, K.; Handzlik, J. MF-8, a novel promising arylpiperazine-hydantoin based 5-HT7 receptor antagonist: In vitro drug-likeness studies and in vivo pharmacological evaluation. Bioorg. Med. Chem. Lett. 2018, 28, 878–883. [Google Scholar] [CrossRef]
- Latacz, G.; Lubelska, A.; Jastrzębska-Więsek, M.; Partyka, A.; Sobiło, A.; Olejarz, A.; Kucwaj-Brysz, K.; Satała, G.; Bojarski, A.J.; Wesołowska, A.; et al. In the search for a lead structure among series of potent and selective hydantoin 5-HT7R agents: The drug-likeness in vitro study. Chem. Biol. Drug Des. 2017, 90, 1295–1306. [Google Scholar] [CrossRef]
- Obach, R.S. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab. Dispos. 1999, 27, 1350–1359. [Google Scholar]
- Cruciani, G.; Carosati, E.; De Boeck, B.; Ethirajulu, K.; Mackie, C.; Howe, T.; Vianello, R. MetaSite: Understanding metabolism in human cytochromes from the perspective of the chemist. J. Med. Chem. 2005, 48, 6970–6979. [Google Scholar] [CrossRef]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Zajdel, P.; Kos, T.; Marciniec, K.; Satała, G.; Canale, V.; Kamiński, K.; Hołuj, M.; Lenda, T.; Koralewski, R.; Bednarski, M.; et al. Novel multi-target azinesulfonamides of cyclic amine derivatives as potential antipsychotics with pro-social and pro-cognitive effects. Eur. J. Med. Chem. 2018, 145, 790–804. [Google Scholar] [CrossRef]
- Kotańska, M.; Śniecikowska, J.; Jastrzębska-Więsek, M.; Kołaczkowski, M.; Pytka, K. Metabolic and Cardiovascular Benefits and Risks of EMD386088—A 5-HT6 Receptor Partial Agonist and Dopamine Transporter Inhibitor. Front. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Kulig, K.; Marcinkowska, M.; Bednarski, M.; Malawska, K.; Zaręba, P. Metabolic benefits of 1-(3-(4-(o-tolyl)piperazin-1-yl)propyl)pyrrolidin-2-one: A non-selective α-adrenoceptor antagonist. J. Endocrinol. Investig. 2018, 41, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, M.; Marcinkowska, M.; Bucki, A.; Olczyk, A.; Kołaczkowski, M. Idalopirdine—A small molecule antagonist of 5-HT6 with therapeutic potential against obesity. Metab. Brain Dis. 2015, 30, 1487–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, M.; Knutelska, J.; Bednarski, M.; Nowiński, L.; Zygmunt, M.; Kazek, G.; Mordyl, B.; Głuch-Lutwin, M.; Zaręba, P.; Kulig, K.; et al. Pyrrolidin-2-one derivatives may reduce body weight in rats with diet-induced obesity. Eur. J. Pharmacol. 2016, 776, 146–155. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds KMP-10 and DJ-18 are available from the authors. |
















| Cpd | Ki (nM) a | ||||
|---|---|---|---|---|---|
| 5-HT6 [3H]-LSD | D2 [3H]-Raclopride | 5-HT1A [3H]-8-OH-DPAT | 5-HT2A [3H]-Ketanserin | 5-HT7 [3H]-5-CT | |
| DJ-18 | 127 | 4098 | 23,300 | nt | 3711 |
| KMP-10 | 87 | 4247 | 14,160 | 17,170 | 514 |
| Ref. | [7] b | [9] b | [20] c | - | [18] d |
| Compound | PAMPA Pe * [10−6 cm/s] ± SD | Caco-2 Papp ** [10−6 cm/s] ± SD | PPB KD *** [μM] | PPB fb *** [%] ± SD | Half-Life t1/2 **** [min] | Intrinsic Clearance CLint **** [mL min−1 kg−1] | |
|---|---|---|---|---|---|---|---|
| DJ-18 | 24.9 ± 0.45 | NT | NT | NT | 187.29 | 4.32 | |
| KMP-10 | 3.76 ± 0.76 | 6.27 ± 0.30 | 112 | 84.5 ± 6.04 | 238.96 | 3.74 | |
| References | Caffeine 15.1 ± 0.40 Norfloxacin 0.56 ± 0.13 | Caffeine 22.04 ± 0.38 | Warfarin 9.50 | Warfarin 98.5 ± 2.10 | Verapamil 30.39 | Verapamil 26.76 |
| Substrate | Molecular Mass (m/z) | Retention Time (min) | Molecular Mass of the Metabolite (m/z) | Metabolic Pathway |
|---|---|---|---|---|
| DJ-18 | 459.3 | 3.99 | M1 445.34 | demethylation |
| 4.21 | M2 475.38 | hydroxylation | ||
| 4.42 | M3 461.36 | demethylation and hydroxylation | ||
| 3.40 | M4 475.38 | hydroxylation | ||
| KMP-10 | 447.27 | 3.45 | M1 433.25 | demethylation |
| 3.65 | M2 463.22 | hydroxylation | ||
| 3.82 | M3 449.26 | demethylation and hydroxylation | ||
| 4.26 | M4 461.23 | hydroxylation and dehydrogenation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lubelska, A.; Latacz, G.; Jastrzębska-Więsek, M.; Kotańska, M.; Kurczab, R.; Partyka, A.; Marć, M.A.; Wilczyńska, D.; Doroz-Płonka, A.; Łażewska, D.; et al. Are the Hydantoin-1,3,5-triazine 5-HT6R Ligands a Hope to a Find New Procognitive and Anti-Obesity Drug? Considerations Based on Primary In Vivo Assays and ADME-Tox Profile In Vitro. Molecules 2019, 24, 4472. https://doi.org/10.3390/molecules24244472
Lubelska A, Latacz G, Jastrzębska-Więsek M, Kotańska M, Kurczab R, Partyka A, Marć MA, Wilczyńska D, Doroz-Płonka A, Łażewska D, et al. Are the Hydantoin-1,3,5-triazine 5-HT6R Ligands a Hope to a Find New Procognitive and Anti-Obesity Drug? Considerations Based on Primary In Vivo Assays and ADME-Tox Profile In Vitro. Molecules. 2019; 24(24):4472. https://doi.org/10.3390/molecules24244472
Chicago/Turabian StyleLubelska, Annamaria, Gniewomir Latacz, Magdalena Jastrzębska-Więsek, Magdalena Kotańska, Rafał Kurczab, Anna Partyka, Małgorzata Anna Marć, Daria Wilczyńska, Agata Doroz-Płonka, Dorota Łażewska, and et al. 2019. "Are the Hydantoin-1,3,5-triazine 5-HT6R Ligands a Hope to a Find New Procognitive and Anti-Obesity Drug? Considerations Based on Primary In Vivo Assays and ADME-Tox Profile In Vitro" Molecules 24, no. 24: 4472. https://doi.org/10.3390/molecules24244472