A New Hyaluronan Modified with β-Cyclodextrin on Hydroxymethyl Groups Forms a Dynamic Supramolecular Network
Abstract
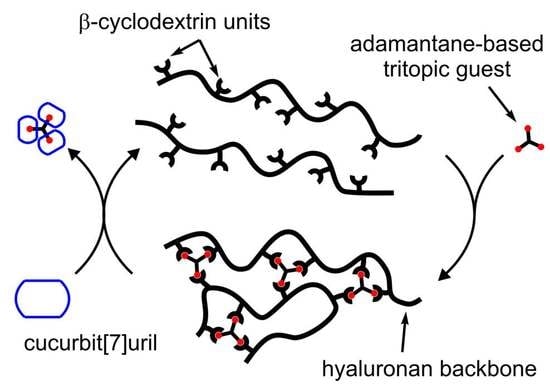
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Characterization of Products
2.3. Supramolecular Study of CD-HA
3. Materials and Methods
3.1. General
3.2. Preparation of 6-O-Monotosyl-6-Deoxy-β-Cyclodextrin (2)
3.3. Preparation of 6-O-Monoazido-6-Deoxy-β-Cyclodextrin (3)
3.4. Preparation of Oxidized Hyaluronan (5)
3.5. Preparation of Propargylated Hyaluronan (6)
3.6. Conjugation of β-CD to HA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rao, M.G.; Bharathi, P.; Akila, R.M. A comprehensive review on biopolymers. Sci. Revs. Chem. Commun. 2014, 4, 61–68. [Google Scholar]
- Bernkop-Schnurch, A.; Dunnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.J.; Ubonvan, T.; Kim, D.D. Hyaluronic acid in drug delivery systems. J. Pharm. Invest. 2010, 40, 33–43. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Hirakura, T.; Yasugi, K.; Nemoto, T. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier new system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wu, C.X.; Huang, J.Y.; Peng, H.X.; Chen, P.; Tang, S.Q. Synthesis and characterization of a degradable composite agarose/HA hydrogel. Carbohydr. Polym. 2012, 88, 1445–1452. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Prestwich, G.D. Hyaluronate derivatives in the drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1998, 15, 513–555. [Google Scholar] [CrossRef]
- Schante, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, H.; Dong, D.; Wang, D.; Zhang, A.; Duan, J. Alteration in immune responses toward N-deacetylation of hyaluronic acid. Glycobiology 2014, 24, 1334–1342. [Google Scholar] [CrossRef] [Green Version]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Crescenzi, V.; Francescangeli, A.; Segre, A.L.; Capitani, D.; Mannina, L.; Renier, D.; Bellini, D. NMR structural study of hydrogels based on partially deacetylated hyaluronan. Macromol. Biosci. 2002, 2, 272–279. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.; Harrington, D.; Farach-Carson, M.; Jia, X. Hyaluronic acid-based hydrogels from a natural polysaccharide to complex networks. Soft Matter 2012, 8, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Segura, T.; Anderson, B.C.; Chung, P.H.; Webber, R.E.; Shull, K.R.; Shea, L.D. Crosslinked hyaluronic acid hydrogels a strategy to functionalize and pattern. Biomaterials 2005, 26, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.K.; Jelacic, S.; Maier, R.V.; Stayton, P.S.; Hoffman, A.S. Anti-inflammatory drug delivery from hyaluronic acid hydrogels. J. Biomater. Sci. Polym. Ed. 2004, 15, 1111–1119. [Google Scholar] [CrossRef]
- Tomihata, K.; Ikada, Y. Cross-linking of hyaluronic acid with glutaraldehyde. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 3553–3559. [Google Scholar] [CrossRef]
- Kuo, J.W.; Swann, D.A.; Prestwich, G.D. Chemical modification of hyaluronic acid by carbodiimides. Bioconjug. Chem. 1991, 2, 232–241. [Google Scholar] [CrossRef]
- Ma, X.; Tian, H. Stimuli-responsive supramolecular polymers in aqueous solution. Acc. Chem. Res. 2014, 47, 1971–1981. [Google Scholar] [CrossRef]
- Dong, R.; Zhou, Y.; Huang, X.; Zhu, X.; Lu, Y.; Shen, J. Functional supramolecular polymers for biomedical applications. Adv. Mater. 2015, 27, 498–526. [Google Scholar] [CrossRef]
- Harada, A. Supramolecular polymers based on cyclodextrins. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5113–5119. [Google Scholar] [CrossRef]
- Rodell, C.B.; Kaminski, A.L.; Burdick, J.A. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 2013, 14, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Li, X.; Ji, F.Y.; Ma, X.; Wang, Q.C.; Tian, H. Photolockable ratiometric viscosity sensitivity of cyclodextrin polypseudorotaxane with light-active rotor graft. Langmuir 2009, 25, 3482–3486. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Chen, J.T.; Liu, Y. Polysaccharide-based noncovalent assembly for targeted delivery of taxol. Sci. Rep. 2016, 6, 19212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhang, Y.M.; Yang, Y.; Chen, L.X.; Liu, Y. Controlled DNA condensation and targeted cellular imaging by ligand exchange in a polysaccharide-quantum dot conjugate. Chem. Commun. 2016, 52, 6087–6090. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; In, I.; Park, S.Y. pH-Responsive NIR-absorbing fluorescent polydopamine with hyaluronic acid for dual targeting and synergistic effects of photothermal and chemotherapy. Biomacromolecules 2017, 18, 1825–1835. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, Y.; Sun, M.; Wu, X.J.; Liu, Y. Construction and drug delivery of a fluorescent TPE-bridged cyclodextrin/hyaluronic acid supramolecular assembles. RSC Adv. 2016, 6, 50673–50679. [Google Scholar] [CrossRef]
- Badwaik, V.; Liu, L.; Gunasekera, D.; Kulkarni, A.; Thompson, D.H. Mechanistic insight into receptor-mediated Delivery of Cationic-β-Cyclodextrin: Hyaluronic Acid-Adamantamethamidyl Host-Guest p-DNA nanoparticles to CD44+ Cells. Mol. Pharm. 2016, 13, 1176–1184. [Google Scholar] [CrossRef]
- Piperno, A.; Zagami, R.; Cordaro, A.; Pennisi, R.; Musarra-Pizzo, M.; Scala, A.; Sciortino, M.T.; Mazzaglia, A. Exploring the entrapment of antiviral agents in hyaluronic acid-cyclodextrin conjugates. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 33–40. [Google Scholar] [CrossRef]
- Banerji, S.; Wright, A.J.; Noble, M.; Mahoney, D.J.; Campbell, I.D.; Day, A.J.; Jackson, D.G. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat. Struct. Mol. Biol. 2007, 14, 234–239. [Google Scholar] [CrossRef]
- Zhong, S.P.; Campoccia, D.; Doherty, P.J.; Williams, R.L.; Benedetti, L.; Williams, D.F. Biodegradation of hyaluronic acid derivatives by hyaluronidase. Biomaterials 1994, 15, 359–365. [Google Scholar] [CrossRef]
- Raoov, M.; Mohamad, S.H.; Radzi, M. Synthesis and characterization of β-cyclodextrin functionalized ionic liquid polymer as a macroporous material for the removal of phenols and As (V). Int. J. Mol. Sci. 2014, 15, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.T.; Wintgens, V.; Amiel, C.; Wimmer, R.; Lambertsen, K. Facile synthesis of β-cyclodextrin-dextran polymers by click chemistry. Biomacromolecules 2010, 11, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Angeles, G.; Němcova, M.; Přikopová, E.; Šmjekalová, D.; Pravda, M.; Kučera, L.; Velebný, V. Reductive alkylation of hyaluronic acid for the synthesis of biocompatible hydrogels by click chemistry. Carbohydr. Polym. 2012, 90, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.; Yang, C.; Rekharsky, M.; Ko, Y.H.; Inoue, Y.; Gilson, M.K. New Ultrahigh Affinity Host-Guest Complexes of Cucurbit[7]uril with Bicyclo[2.2.2]octane and Adamantane Guests: Thermodynamic Analysis and Evaluation of M2 Affinity Calculations. J. Am. Chem. Soc. 2011, 133, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Branná, P.; Rouchal, M.; Prucková, Z.; Dastychová, L.; Lenobel, R.; Pospíšil, T.; Maláč, K.; Vícha, R. Rotaxanes capped with host molecules: Supramolecular behavior of adamantylated bisimidazolium salts containing a biphenyl centerpiece. Chem. Eur. J. 2015, 21, 11712–11718. [Google Scholar]
- Kulkarni, S.G.; Prucková, Z.; Rouchal, M.; Dastychová, L.; Vícha, R. Adamantylated trisimidazolium-based tritopic guests and their binding properties towards cucurbit[7]uril and β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 11–20. [Google Scholar] [CrossRef]
- Schneider, H.-J.; Hacket, F.; Rüdiger, V.; Ikeda, H. NMR Studies of Cyclodextrins and Cyclodextrin Complexes. Chem. Rev. 1998, 98, 1755–1785. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. Cucurbiturils: From synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 2015, 44, 394–418. [Google Scholar] [CrossRef]
- Barrow, S.J.; Kasera, S.; Rowland, M.J.; del Barrio, J.; Scherman, O.A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |






| Guest | Host | n1 | K2 [dm3·mol−1] | −ΔH [kJ·mol−1] | ΔS [J·mol−1·K−1] |
|---|---|---|---|---|---|
| G1 | β-CD | 0.96 | 7.05 × 103 | 24.5 | −7 |
| CB7 3 | ng 4 | 1.7 × 1014 | 80.6 | 0 | |
| CD-HA | 1 | 1.12 × 104 | 13.9 | 32 | |
| G2 | β-CD 5 | 0.49 | 9.20 × 104 | 59.0 | −99 |
| CB7 5 | 0.56 | 1.35 × 1012 | ng | ng | |
| CD-HA | 0.43 | 1.14 × 106 | 11.4 | 79 | |
| G3 | β-CD 6 | 0.37 | 1.02 × 105 | 85.7 | −187 |
| CB7 6 | 0.39 | 4.84 × 1010 | 173.8 | −369 | |
| CD-HA | 0.31 | 1.00 × 105 | 87.6 | −193 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, J.; Prucková, Z.; Pospíšil, T.; Kašpárková, V.; Rouchal, M.; Vícha, R. A New Hyaluronan Modified with β-Cyclodextrin on Hydroxymethyl Groups Forms a Dynamic Supramolecular Network. Molecules 2019, 24, 3849. https://doi.org/10.3390/molecules24213849
Kovačević J, Prucková Z, Pospíšil T, Kašpárková V, Rouchal M, Vícha R. A New Hyaluronan Modified with β-Cyclodextrin on Hydroxymethyl Groups Forms a Dynamic Supramolecular Network. Molecules. 2019; 24(21):3849. https://doi.org/10.3390/molecules24213849
Chicago/Turabian StyleKovačević, Jelica, Zdeňka Prucková, Tomáš Pospíšil, Věra Kašpárková, Michal Rouchal, and Robert Vícha. 2019. "A New Hyaluronan Modified with β-Cyclodextrin on Hydroxymethyl Groups Forms a Dynamic Supramolecular Network" Molecules 24, no. 21: 3849. https://doi.org/10.3390/molecules24213849