TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties
Abstract
:1. Introduction
2. Results
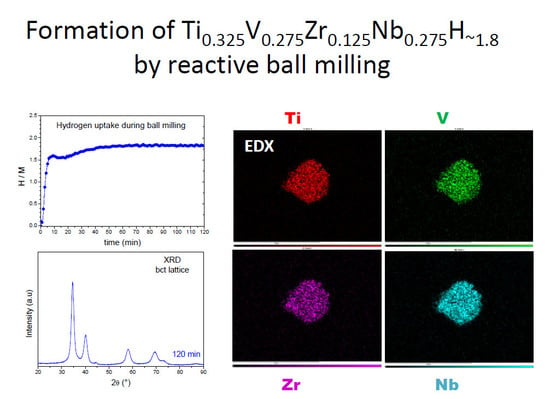
2.1. Synthesis of the TiVZrNb Alloy and Related Hydride
2.2. Crystalline Structure of the TiVZrNb Alloy and Related Hydride
2.3. Hydrogen Sorption Properties of the TiVZrNb Alloy Prepared by BM
2.4. Hydrogen Sorption Properties of the TiVZrNb Alloy Prepared by HT-AM
2.5. Hydrogen Sorption Properties of the TiVZrNbH1.8 Hydride Prepared by RBM
2.6. SQS-DFT Modeling
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys-A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Gao, M.C.; Miracle, D.B.; Maurice, D.; Yan, X.; Zhang, Y.; Hawk, J.A. High-entropy functional materials. J. Mater. Res. 2018, 1–18. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int. J. Hydrog. Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Sahlberg, M.; Karlsson, D.; Zlotea, C.; Jansson, U. Superior hydrogen storage in high entropy alloys. Sci. Rep. 2016, 6, 36770. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Zhang, J.; Hu, J.; Zhang, J.; Mao, Y.; Xiao, H.; Zhou, X.; Zu, X. A Novel TiZrHfMoNb High-Entropy Alloy for Solar Thermal Energy Storage. Nanomaterials 2019, 9, 248. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.-P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J. Alloy. Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sahlberg, M.; Sørby, M.H.; Hauback, B.C. Hydrogen storage in high-entropy alloys with varying degree of local lattice strain. Int. J. Hydrog. Energy 2019. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; You, L.; Cao, X.; Lu, Z.; Song, X. Investigation on the activation mechanism of hydrogen absorption in TiZrNbTa high entropy alloy. J. Alloy. Compd. 2019, 781, 613–620. [Google Scholar] [CrossRef]
- Karlsson, D.; Ek, G.; Cedervall, J.; Zlotea, C.; Møller, K.T.; Hansen, T.C.; Bednarčík, J.; Paskevicius, M.; Sørby, M.H.; Jensen, T.R.; et al. Structure and Hydrogenation Properties of a HfNbTiVZr High-Entropy Alloy. Inorg. Chem. 2018, 57, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting electrons - A new approach to tailor the hydrogen sorption properties of high-entropy alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. Hig-Entropy Alloys Fundamentals and Applications; Springer: Berlin, Germany, 2016; Vol. chapter 10; ISBN 978-3-319-27011-1. [Google Scholar]
- Tian, F. A Review of Solid-Solution Models of High-Entropy Alloys Based on Ab Initio Calculations. Front. Mater. 2017, 4, 36. [Google Scholar] [CrossRef]
- King, D.J.M.; Cheung, S.T.Y.; Humphry-Baker, S.A.; Parkin, C.; Couet, A.; Cortie, M.B.; Lumpkin, G.R.; Middleburgh, S.C.; Knowles, A.J. High temperature, low neutron cross-section high-entropy alloys in the Nb-Ti-V-Zr system. Acta Mater. 2019, 166, 435–446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cuevas, F.; Zaidi, W.; Bonnet, J.-P.; Aymard, L.; Bobet, J.-L.; Latroche, M. Highlighting of a Single Reaction Path during Reactive Ball Milling of Mg and TM by Quantitative H-2 Gas Sorption Analysis To Form Ternary Complex Hydrides (TM = Fe, Co, Ni). J. Phys. Chem. C 2011, 115, 4971–4979. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1998, 6, 461–470. [Google Scholar] [CrossRef]
- Akiba, E.; Okada, M. Metallic Hydrides III: Body-Centered-Cubic Solid-Solution Alloys. MRS Bull. 2002, 27, 699–703. [Google Scholar] [CrossRef]
- Zepon, G.; Leiva, D.R.; Strozi, R.B.; Bedoch, A.; Figueroa, S.J.A.; Ishikawa, T.T.; Botta, W.J. Hydrogen-induced phase transition of MgZrTiFe0.5Co0.5Ni0.5 high entropy alloy. Int. J. Hydrog. Energy 2018, 43, 1702–1708. [Google Scholar] [CrossRef]
- Klebanoff, L. Hydrogen Storage Technology: Materials and Applications; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-4107-5. [Google Scholar]
- Doppiu, S.; Schultz, L.; Gutfleisch, O. In situ pressure and temperature monitoring during the conversion of Mg into MgH2 by high-pressure reactive ball milling. J. Alloy. Compd. 2007, 427, 204–208. [Google Scholar] [CrossRef]
- Zlotea, C.; Chevalier-Cesar, C.; Leonel, E.; Leroy, E.; Cuevas, F.; Dibandjo, P.; Vix-Guterl, C.; Martens, T.; Latroche, M. Synthesis of small metallic Mg-based nanoparticles confined in porous carbon materials for hydrogen sorption. Faraday Discuss. 2011, 151, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B: Condens. Matter 1993, 192, 55–69. [Google Scholar]
- Zunger, A.; Wei, S.H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, A.; Tiwary, P.; de Jong, M.; Olmsted, D.L.; Asta, M.; Dick, A.; Shin, D.; Wang, Y.; Chen, L.-Q.; Liu, Z.-K. Efficient stochastic generation of special quasirandom structures. Calphad 2013, 42, 13–18. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Erratum: Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
Sample Availability: All samples are available from the authors. |








| Sample | Preparation Method/Modeling | Phase | Structure | Space Group | Lattice Parameters (Å) |
|---|---|---|---|---|---|
| Ti0.325V0.275Zr0.125Nb0.275 | HT-AM | alloy | bcc | Im-3m | a = 3.261(1) |
| Ti0.325V0.275Zr0.125Nb0.275H1.75 | hydride | bct | I4/mmm | a = 3.168(1) c = 4.523(1) | |
| Ti0.325V0.275Zr0.125Nb0.275 | desorbed | bcc | Im-3m | a = 3.253(1) | |
| Ti0.325V0.275Zr0.125Nb0.275 | BM | alloy | bcc | Im-3m | a = 3.270(1) |
| Ti0.325V0.275Zr0.125Nb0.275H1.7 | hydride | bct | I4/mmm | a = 3.167(1) c = 4.371(1) | |
| Ti0.325V0.275Zr0.125Nb0.275 | desorbed | bcc | Im-3m | a = 3.268(1) | |
| Ti0.325V0.275Zr0.125Nb0.275H1.8 | RBM | hydride | bct | I4/mmm | a = 3.194(1) c = 4.448(1) |
| Ti0.325V0.275Zr0.125Nb0.275 | desorbed | bcc | Im-3m | a = 3.277(1) | |
| TiVZrNb | SQS-DFT | alloy | bcc | Im-3m | a = 3.29 |
| TiVZrNbH2 | hydride | bct | I4/mmm | a = 3.19 c = 4.52 | |
| TiVZrNbH2 | hydride | fcc | Fm-3m | a = 4.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, J.; Zlotea, C.; Ek, G.; Crivello, J.-C.; Laversenne, L.; Sahlberg, M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules 2019, 24, 2799. https://doi.org/10.3390/molecules24152799
Montero J, Zlotea C, Ek G, Crivello J-C, Laversenne L, Sahlberg M. TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties. Molecules. 2019; 24(15):2799. https://doi.org/10.3390/molecules24152799
Chicago/Turabian StyleMontero, Jorge, Claudia Zlotea, Gustav Ek, Jean-Claude Crivello, Lætitia Laversenne, and Martin Sahlberg. 2019. "TiVZrNb Multi-Principal-Element Alloy: Synthesis Optimization, Structural, and Hydrogen Sorption Properties" Molecules 24, no. 15: 2799. https://doi.org/10.3390/molecules24152799