Assessment of the Bulgarian Wastewater Treatment Plants’ Impact on the Receiving Water Bodies
Abstract
:1. Introduction
2. Results
2.1. Sampling and Basic Statistics
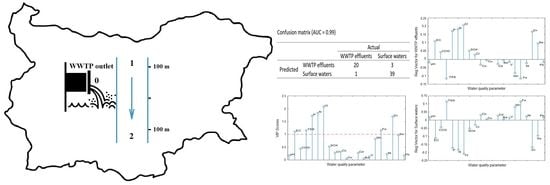
2.2. PLS-DA Models
3. Discussion
4. Materials and Methods
4.1. Sampling and Sample Preparation
4.2. Physicochemical Analysis
4.2.1. Spectrophotometric Methods Using Cuvette Tests
4.2.2. ICP-MS
4.3. Ecotoxicological Analysis
4.4. PLS-DA
5. Conclusions
- A new way for WWTPs’ impact assessment on receiving water bodies;
- Prioritization of water quality indicators concerning WWTPs’ impact on receiving water bodies;
- Opportunity for selection of optimal water quality indicator set for assessment of WWTPs’ impact.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chutter, F. Research on the Rapid Biological Assessment of Water Quality Impacts in Streams and Rivers; WRC Report No 422/1/98; Water Research Commission: Pretoria, South Africa, 1998. [Google Scholar]
- Cantinho, P.; Matos, M.; Trancoso, M.A.; Correia dos Santos, M.M. Behaviour and fate of metals in urban wastewater treatment plants: A review. Int. J. Environ. Sci. Technol. 2016, 13, 359–386. [Google Scholar] [CrossRef]
- Barreca, S.; Busetto, M.; Vitelli, M.; Colzani, L.; Clerici, L.; Dellavedova, P. Online Solid-Phase Extraction LC-MS/MS: A Rapid and Valid Method for the Determination of Perfluorinated Compounds at Sub ng·L−1 Level in Natural Water. J. Chem. 2018, 2018, 3780825. [Google Scholar] [CrossRef]
- Barreca, S. Determination of estrogenic endocrine disruptors in water at sub-ng L−1 levels in compliance with Decision 2015/495/EU using offline-online solid phase extraction concentration coupled with high performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 147, 1186–1191. [Google Scholar] [CrossRef]
- Victor, R.; Kotter, R.; O’Brien, G.; Mitropoulos, M.; Panayi, G. WHO Guidelines for the safe use of wastewater, excreta and greywater—Volume 1: Policy and regulatory aspects. Int. J. Environ. Stud. 2006, 65, 157–176. [Google Scholar] [CrossRef]
- The Millennium Development Goals Report; The United Nation: New York, NY, USA, 2015.
- European Parliament, Council of the European Union. Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy. OJ. L. 2000, 327, 1–73. [Google Scholar]
- Council of the European Union. Council Directive 91/271/EEC concerning urban waste-water treatment. OJ. L. 1991, 135, 40–52. [Google Scholar]
- Council of the European Union. Council Directive 98/83/EC on the quality of water intended for human consumption. OJ. L. 1998, 330, 32–54. [Google Scholar]
- Teodosiu, C.; Barjoveanu, G.; Teleman, D. Sustainable water resources management 1. River basin management and the EC Water Framework Directive. Environ. Eng. Manag. J. 2003, 2, 377–394. [Google Scholar] [CrossRef]
- Johnson, C. Toward post-sovereign environmental governance? Politics, scale, and EU water framework directive. Water Altern. 2012, 5, 83–97. [Google Scholar]
- Carter, J. Spatial planning, water and the Water Framework Directive: Insights from theory and practice. Geogr. J. 2007, 173, 330–342. [Google Scholar] [CrossRef]
- Josefsson, H. Achieving ecological objectives. Laws 2012, 1, 39–63. [Google Scholar] [CrossRef]
- Van Rijswick, H.; Backes, C. Ground breaking landmark case on environmental quality standards? J. Eur. Environ. Plan. Law 2015, 12, 363–377. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 75/440/EEC concerning the quality required of surface water intended for the abstraction of drinking water in the Member States. OJ. L. 1975, 194, 26–31. [Google Scholar]
- Junninen, H.; Mønster, J.; Rey, M.; Cancelinha, J.; Douglas, K.; Duane, M.; Forcina, V.; Müller, A.; Lagler, F.; Marelli, L.; et al. Quantifying the impact of residential heating on the urban air quality in a typical European coal combustion region. Environ. Sci. Technol. 2009, 43, 7964–7970. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S.; Singh, V.K. Chemometric data analysis of pollutants in wastewater—A case study. Anal. Chim. Acta 2005, 532, 15–25. [Google Scholar] [CrossRef]
- Einax, J.W.; Soldt, U. Geostatistical and multivariate statistical methods for the assessment of polluted soils—merits and limitations. Chemom. Intell. Lab. Syst. 1999, 46, 79–91. [Google Scholar] [CrossRef]
- Barreca, S.; Mazzola, A.; Orecchio, S.; Tuzzolino, N. Polychlorinated Biphenyls in Sediments from Sicilian Coastal Area (Scoglitti) using Automated Soxhlet, GC-MS, and Principal Component Analysis. Polycycl. Aromat. Comp. 2014, 34, 237–262. [Google Scholar] [CrossRef]
- Gurjar, S.K.; Tare, V. Spatial-temporal assessment of water quality and assimilative capacity of river Ramganga, a tributary of Ganga using multivariate analysis and QUEL2K. J. Clean. Prod. 2019, 222, 550–564. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Liang, C.; Bush, R.T.; Xiong, L.; Jiang, Y. A comparative assessment of Australia’s Lower Lakes water quality under extreme drought and post-drought conditions using multivariate statistical techniques. J. Clean. Prod. 2018, 190, 1–11. [Google Scholar] [CrossRef]
- Bilgin, A. Evaluation of surface water quality by using Canadian Council of Ministers of the Environment Water Quality Index (CCME WQI) method and discriminant analysis method: A case study Coruh River Basin. Environ. Monit. Assess. 2018, 190, 554. [Google Scholar] [CrossRef]
- Singh, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—A case study. Water Res. 2004, 38, 3980–3992. [Google Scholar] [CrossRef] [PubMed]
- Astel, A.; Tsakovski, S.; Barbieri, P.; Simeonov, V. Comparison of self-organizing maps classification approach with cluster and principal components analysis for large environmental data sets. Water Res. 2007, 41, 4566–4578. [Google Scholar] [CrossRef] [PubMed]
- Olkowska, E.; Kudłak, B.; Tsakovski, S.; Ruman, M.; Simeonov, V.; Polkowska, Z. Assessment of the water quality of Kłodnica River catchment using self-organizing maps. Sci. Total Environ. 2014, 476–477, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Acquavita, A.; Aleffi, I.F.; Benci, C.; Bettoso, N.; Crevatin, E.; Milani, L.; Tamberlich, F.; Toniatti, L.; Barbieri, P.; Licen, S.; et al. Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: The Marano and Grado Lagoon (northern Adriatic Sea). Reg. Stud. Mar. Sci. 2015, 2, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Franklin, J.B.; Sathish, T.; Vinithkumar, N.V.; Kirubagaran, R.; Madeswaran, P. Seawater quality conditions of the south Andaman Sea (Bay of Bengal, Indian Ocean) in lustrum during 2010s decade. Mar. Pollut. Bull. 2018, 136, 424–434. [Google Scholar] [CrossRef]
- Bostanmaneshrad, F.; Partani, S.; Noori, R.; Nachtnebel, H.-P.; Berndtsson, R.; Adamowski, J.F. Relationship between water quality and macro-scale parameters (land use, erosion, geology, and population density) in the Siminehrood River Basin. Sci. Total Environ. 2018, 639, 1588–1600. [Google Scholar] [CrossRef]
- Voyslavov, T.; Tsakovski, S.; Simeonov, V. Hasse diagram technique as a tool for water quality assessment. Anal. Chim. Acta 2013, 770, 29–35. [Google Scholar] [CrossRef]
- Voyslavov, T.; Tsakovski, S.; Simeonov, V. Surface water quality assessment using self-organizing maps and Hasse diagram technique. Chemom. Intell. Lab. Syst. 2012, 118, 280–286. [Google Scholar] [CrossRef]
- Tsakovski, S.; Astel, A.; Simeonov, V. Assessment of the water quality of a river catchment by chemometric expertise. J. Chemom. 2010, 24, 694–702. [Google Scholar] [CrossRef]
- Singh, K.R.; Goswami, A.P.; Kalamdhad, A.S.; Kumar, B. Assessment of surface water quality of Pagladia, Beki and Kolong river (Assam, India) using multivariate statistical techniques. Int. J. River Basin Manag. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Liong, C.-Y.; Jemain, A.A. Partial Least Squares-Discriminant Analysis (PLS-DA) for classification of high-dimensional (HD) data: A review of contemporary practice strategies and knowledge gaps. Analyst 2018, 143, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Platikanov, S.; Rodriguez-Mozaz, S.; Huerta, B.; Barceló, D.; Cros, J.; Batle, M.; Poch, G.; Tauler, R. Chemometrics quality assessment of wastewater treatment plant effluents using physicochemical parameters and UV absorption measurements. J. Environ. Manag. 2014, 140, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Tobiszewski, M.; Tsakovski, S.; Simeonov, V.; Namiesnik, J. Chlorinated solvents in a petrochemical wastewater treatment plant: An assessment of their removal using self-organising maps. Chemosphere 2012, 87, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M.; Yotova, G.; Tsakovski, S.; Simeonov, V.; Namiesnik, J. Environmental risk assessment of Polish wastewater treatment plant activity. Chemosphere 2016, 160, 181–188. [Google Scholar] [CrossRef]
- Manusadzianas, L.; Balkelyte, L.; Sadauskas, K.; Blinova, I.; Põllumaa, L.; Kahru, A. Ecotoxicological study of Lithuanian and Estonian wastewaters: Selection of the biotests, and correspondence between toxicity and chemical-based indices. Aquat. Toxicol. 2003, 63, 27–41. [Google Scholar] [CrossRef]
- Wang, N.; Zeng, N.N.; Zhu, W. Sensitivity, Specificity, Accuracy, Associated Confidence Interval and ROC Analysis with Practical SAS Implementations. In Proceedings of the Northeast SAS User Group Section of Health Care and Life Sciences, Baltimore, MD, USA, 14–17 November 2010; pp. 1–9. [Google Scholar]
- Tjandraatmadja, G.; Pollard, C.; Sheedy, C.; Gozukara, Y. Sources of Contaminants in Domestic Wastewater: Nutrients and Additional Elements from Household Products; CSIRO Publishing: Canberra, Australia, 2010. [Google Scholar]
- European Commission. Pollutants in Urban Wastewater and Sewage Sludge; Office for Official Publications of the European Communities: Luxembourg, 2001; pp. 12–63. [Google Scholar]
- DIN 38409-41:1980-12—German standard methods for the examination of water, waste water and sludge; Summary effect and substance characteristics (group H); Determination of the chemical oxygen demand (COD) in the range above 15 mg/L (H 41). German Institute for Standardisation, 1980. [CrossRef]
- Hach Company. Working Procedure: LCK 138 LATON, 1–16 mg/L Total Nitrogen, TNb DOC312.53.94004. Available online: https://uk.hach.com/asset-get.download.jsa?id=52788795653 (accessed on 9 June 2019).
- Hach Company. Working procedure: LCK348 Phosphate DOC312.53.94020. Available online: https://uk.hach.com/asset-get.download.jsa?id=25593618013 (accessed on 9 June 2019).
- Hach Company. User Manual: 5014 Probe DOC012.98.90299. Available online: https://uk.hach.com/asset-get.download.jsa?id=25593610956 (accessed on 9 June 2019).
- Hach Company. User Manual: 5070 Probe DOC012.98.90314. Available online: https://uk.hach.com/asset-get.download.jsa?id=25593610971 (accessed on 9 June 2019).
- BSI. BS EN 872:2005—Water Quality—Determination of Suspended Solids—Method by Filtration Through Glass Fibre Filters. BSI, 2005. [Google Scholar]
- Wieczerzak, M.; Kudłak, B.; Namieśnik, J. Impact of selected drugs and their binary mixtures on the germination of Sorghum bicolor (sorgo) seeds. Environ. Sci. Pollut. Res. 2018, 25, 18717–18727. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, S3–S16. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |




| Parameter | Unit | Mean | Min | Max | St. dev. | Directive 91/271/EEC | Samples Exceeding (n) |
|---|---|---|---|---|---|---|---|
| pH | - | 8.13 | 7.57 | 8.51 | 0.29 | - | - |
| EC | µS/cm | 451.7 | 87.30 | 1174 | 275.4 | - | - |
| COD | mg/L O2 | 12.13 | 5.69 | 23.40 | 4.27 | 125 | - |
| TSS | mg/L | 3.26 | 0.10 | 9.40 | 2.19 | 35/60 1 | - |
| P | mg/L | 1.14 | <0.50 | 2.82 | 0.73 | ½ 2 | 5 |
| N | mg/L | 7.07 | 1.85 | 14.20 | 3.41 | 10/15 2 | 1 |
| Cl− | mg/L | 42.2 | 17.4 | 86.6 | 19.6 | - | - |
| SO42− | mg/L | 53 | 5 | 136 | 37 | - | - |
| Cr | mg/L | 0.0026 | 0.0005 | 0.0139 | 0.0027 | - | - |
| Co | mg/L | 0.0002 | 0.0001 | 0.0005 | 0.0001 | - | - |
| Cu | mg/L | 0.0023 | 0.0007 | 0.0057 | 0.0015 | - | - |
| Cd | µg/L | 0.0113 | 0.0001 | 0.0918 | 0.0270 | - | - |
| Ba | mg/L | 0.0272 | 0.0131 | 0.0560 | 0.0132 | - | - |
| V | mg/L | 0.0015 | 0.0006 | 0.0064 | 0.0013 | - | - |
| Mn | mg/L | 0.0329 | 0.0043 | 0.1146 | 0.0300 | - | - |
| Fe | mg/L | 0.239 | 0.093 | 0.408 | 0.085 | - | - |
| Ni | mg/L | 0.0028 | 0.0016 | 0.0097 | 0.0018 | - | - |
| Zn | mg/L | 0.0222 | 0.0056 | 0.0618 | 0.0162 | - | - |
| Se | mg/L | 0.0006 | 0.0000 | 0.0026 | 0.0006 | - | - |
| Pb | mg/L | 0.0010 | 0.0001 | 0.0144 | 0.0031 | - | - |
| LS-SG | % | 3.02 | 0.00 | 6.67 | 2.27 | - | - |
| LS-RG | % | −30.59 | −92.99 | 33.83 | 27.54 | - | - |
| SA-SG | % | 0.16 | −3.45 | 10.34 | 4.00 | - | - |
| SA-RG | % | −26.15 | −69.55 | 27.33 | 25.56 | - | - |
| SS-SG | % | 1.31 | −3.45 | 6.90 | 3.53 | - | - |
| SS-RG | % | 14.65 | −4.71 | 40.18 | 12.58 | - | - |
| Daphnia | % | 18.10 | 0.00 | 46.67 | 11.76 | - | - |
| Microtox | % | 27.53 | −14.36 | 61.81 | 18.90 | - | - |
| Directive 75/440/EEC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Unit | Mean | Min | Max | St. dev. | A1 | A2 | A3 | Samples Exceeding A1 (n) |
| pH | - | 8.16 | 7.54 | 8.53 | 0.25 | 6.5–8.5 | 5.5–9.0 | 5.5–9.0 | 1 |
| EC | µS/cm | 269.5 | 26.90 | 607.0 | 162.6 | 1000 | 1000 | 1000 | - |
| COD | mg/L O2 | 9.51 | <5.00 | 59.20 | 9.19 | - | - | 30 | 2 |
| TSS | mg/L | 10.91 | 1.80 | 40.30 | 9.94 | 25 | - | - | 6 |
| P | mg/L | 0.38 | <0.50 | 1.37 | 0.29 | 0.4 | 0.7 | 0.7 | 8 |
| N | mg/L | 2.58 | <1.00 | 9.90 | 2.01 | 1 | 2 | 3 | 36 |
| Cl− | mg/L | 13.0 | 1.6 | 39.4 | 8.2 | 200 | 200 | 200 | - |
| SO42− | mg/L | 39 | 7 | 230 | 38 | 150 | 150 | 150 | - |
| Cr 1 | mg/L | 0.0021 | 0.0003 | 0.0097 | 0.0025 | 0.05 | 0.05 | 0.05 | - |
| Co | mg/L | 0.0003 | 0.0001 | 0.0006 | 0.0001 | 0.02 | - | - | - |
| Cu | mg/L | 0.0024 | 0.0006 | 0.0098 | 0.0019 | 0.02 | 0.05 | 1 | - |
| Cd | µg/L | 0.0199 | 0.0001 | 0.2256 | 0.0464 | 1 | 1 | 1 | - |
| Ba 1 | mg/L | 0.0277 | 0.0067 | 0.0531 | 0.0124 | 0.1 | 1 | 1 | - |
| V | mg/L | 0.0016 | 0.0004 | 0.0043 | 0.0011 | 0.01 | - | - | - |
| Mn | mg/L | 0.0508 | 0.0072 | 0.1182 | 0.0275 | 0.05 | 0.1 | 1 | 18 |
| Fe | mg/L | 0.388 | 0.128 | 0.885 | 0.186 | 0.1 | 1 | 1 | 42 |
| Ni | mg/L | 0.0031 | 0.0012 | 0.0062 | 0.0012 | 0.02 | - | - | - |
| Zn | mg/L | 0.0069 | 0.0013 | 0.0209 | 0.0045 | 0.5 | 1 | 1 | - |
| Se 1 | mg/L | 0.0003 | 0.0000 | 0.0015 | 0.0003 | 0.01 | 0.01 | 0.01 | - |
| Pb 1 | mg/L | 0.0013 | 0.0001 | 0.0068 | 0.0018 | 0.05 | 0.05 | 0.05 | - |
| LS-SG | % | 2.26 | 0.00 | 10.00 | 2.85 | - | - | - | - |
| LS-RG | % | −24.82 | −83.91 | 63.89 | 33.87 | - | - | - | - |
| SA-SG | % | 0.04 | −3.45 | 10.34 | 3.58 | - | - | - | - |
| SA-RG | % | −16.72 | −68.26 | 27.69 | 27.61 | - | - | - | - |
| SS-SG | % | 0.00 | −3.45 | 10.34 | 3.73 | - | - | - | - |
| SS-RG | % | 13.28 | −9.43 | 45.83 | 14.99 | - | - | - | - |
| Daphnia | % | 19.37 | 0.00 | 40.00 | 9.75 | - | - | - | - |
| Microtox | % | 27.29 | −16.69 | 63.41 | 22.01 | - | - | - | - |
| Instrument | Operating Conditions |
|---|---|
| Argon plasma gas flow | 15 L/min |
| Auxiliary gas flow | 1.20 L/min |
| Nebulizer gas flow | 0.90 L/min |
| Lens voltage | 6.00 V |
| ICP RF power | 1100 W |
| Pulse stage voltage | 950 V |
| Dwell time | 50 ms |
| Acquisition mode | Peak hop |
| Peak pattern | One point per mass at maximum peak |
| Sweeps/reading | 8 |
| Reading/replicates | 1 |
| Sample uptake rate | 2 mL/min |
| Number of runs | 6 |
| Rinse time | 180 s |
| Rinse solution | 3% HNO3 (v/v) |
| Isotopes monitored | 137Ba, 111Cd, 59Co, 52Cr, 63Cu, 57Fe, 55Mn, 60Ni, 208Pb, 78Se, 51V, 66Zn |
| Actual | |||
|---|---|---|---|
| Class A | Class B | ||
| Predicted | Class A | TP | FN |
| Class B | FP | TN | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yotova, G.; Lazarova, S.; Kudłak, B.; Zlateva, B.; Mihaylova, V.; Wieczerzak, M.; Venelinov, T.; Tsakovski, S. Assessment of the Bulgarian Wastewater Treatment Plants’ Impact on the Receiving Water Bodies. Molecules 2019, 24, 2274. https://doi.org/10.3390/molecules24122274
Yotova G, Lazarova S, Kudłak B, Zlateva B, Mihaylova V, Wieczerzak M, Venelinov T, Tsakovski S. Assessment of the Bulgarian Wastewater Treatment Plants’ Impact on the Receiving Water Bodies. Molecules. 2019; 24(12):2274. https://doi.org/10.3390/molecules24122274
Chicago/Turabian StyleYotova, Galina, Svetlana Lazarova, Błażej Kudłak, Boika Zlateva, Veronika Mihaylova, Monika Wieczerzak, Tony Venelinov, and Stefan Tsakovski. 2019. "Assessment of the Bulgarian Wastewater Treatment Plants’ Impact on the Receiving Water Bodies" Molecules 24, no. 12: 2274. https://doi.org/10.3390/molecules24122274