Antihypertensive Effects of Corn Silk Extract and Its Novel Bioactive Constituent in Spontaneously Hypertensive Rats: The Involvement of Angiotensin-Converting Enzyme Inhibition
Abstract
:1. Introduction
2. Results
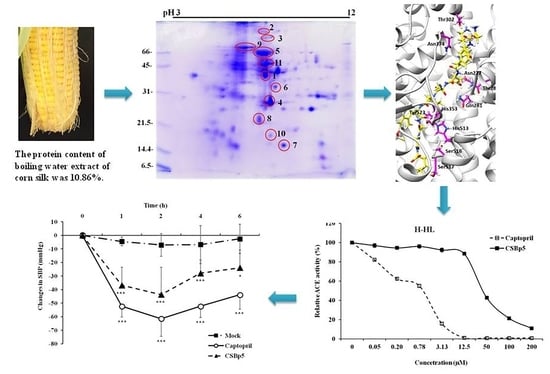
2.1. CSE Reduced Blood Pressure In Vivo and Inhibited ACE Activity In Vitro
2.2. Identification of Bioactive Peptides with Blood Pressure Lowering-Potentials in CSE
2.3. CSBp5 Inhibited ACE Activity In Vitro and Reduced Blood Pressure In Vivo
2.4. Interaction between CSBp5 and ACE by Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Materials and Extraction Procedure
4.3. Animal Experiments
4.4. ACE Activity Assay
4.5. 2-DE and LC-MS/MS Analysis
4.6. Bioinformatics Analysis
4.7. Preparation of Synthetic Peptides
4.8. Docking Analysis
4.9. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef]
- Bader, M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 439–465. [Google Scholar] [CrossRef]
- Watermeyer, J.M.; Kroger, W.L.; Sturrock, E.D.; Ehlers, M.R.W. Angiotensin converting enzyme—New insights into structure, biological significance and prospects for domain-selective inhibitors. Curr. Enzym. Inhib. 2009, 5, 134–147. [Google Scholar] [CrossRef]
- Wright, J.M.; Musini, V.M.; Gill, R. First-line drugs for hypertension. Cochrane Database Syst. Rev. 2008, 4, CD001841. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Antihypertensive peptides of animal origin: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 566–578. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Joel, C.H.; Sutopo, C.C.Y.; Prajitno, A.; Su, J.H.; Hsu, J.L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Qiao, L.; Li, L.; Zhang, Y.; Li, K.; Wang, L.; Qiao, Y. A novel antihypertensive derived from adlay (Coix larchryma-jobi L. var. ma-yuen Stapf) glutelin. Molecules 2017, 22, 123. [Google Scholar] [CrossRef]
- Ramírez-Torres, G.; Ontiveros, N.; Lopez-Teros, V.; Ibarra-Diarte, J.; Reyes-Moreno, C.; Cuevas-Rodríguez, E.O.; Cabrera-Chávez, F. Amaranth protein hydrolysates efficiently reduce systolic blood pressure in spontaneously hypertensive rats. Molecules 2017, 22, 1905. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.B.; Yamamoto, A.; Matsumoto, S.; Ito, H.; Igami, K.; Miyazaki, T.; Kondo, R.; Shimizu, K. Hypotensive effects and angiotensin-converting enzyme inhibitory peptides of reishi (Ganoderma lingzhi) auto-digested extract. Molecules 2014, 19, 13473–13485. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Okubo, A.; Yamazaki, S.; Takano, T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J. Dairy Sci. 1995, 78, 777–783. [Google Scholar] [CrossRef]
- Ishida, Y.; Shibata, Y.; Fukuhara, I.; Yano, Y.; Takehara, I.; Kaneko, K. Effect of an excess intake of casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro in subjects with normal blood pressure, high-normal blood pressure, or mild hypertension. Biosci. Biotechnol. Biochem. 2011, 75, 427–433. [Google Scholar] [CrossRef]
- Shi, S.; Li, S.; Li, W.; Xu, H. Corn silk tea for hypertension: A systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2019, 2019, 2915498. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xiao, T.; Ruan, J.; Liu, W. Beneficial effects of corn silk on metabolic syndrome. Curr. Pharm. Des. 2017, 23, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Ma, Z.; Cheng, J.; Liu, J. Anti-diabetic, anti-oxidant and anti-hyperlipidemic activities of flavonoids from corn silk on STZ-induced diabetic mice. Molecules 2016, 21, 7. [Google Scholar] [CrossRef]
- Peng, K.Z.; Yang, X.; Zhou, H.L.; Pan, S.X. Safety evaluation, in vitro and in vivo antioxidant activity of the flavonoid-rich extract from Maydis stigma. Molecules 2015, 20, 22102–22112. [Google Scholar] [CrossRef] [PubMed]
- Sabiu, S.; O’Neill, F.H.; Ashafa, A.O.T. Kinetics of α-amylase and α-glucosidase inhibitory potential of Zea mays Linnaeus (Poaceae), Stigma maydis aqueous extract: An in vitro assessment. J. Ethnopharmacol. 2016, 183, 1–8. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, C.; Chen, Z.; Li, W.; Yuan, G.; Chen, H. Physicochemical properties and antidiabetic effects of a polysaccharide from corn silk in high-fat diet and streptozotocin-induced diabetic mice. Carbohydr. Polym. 2017, 164, 370–378. [Google Scholar] [CrossRef]
- Ho, T.Y.; Li, C.C.; Lo, H.Y.; Chen, F.Y.; Hsiang, C.Y. Corn silk extract and its bioactive peptide ameliorated lipopolysaccharide-induced inflammation in mice via the nuclear factor-κB signaling pathway. J. Agric. Food Chem. 2017, 65, 759–768. [Google Scholar] [CrossRef]
- George, G.O.; Idu, F.K. Corn silk aqueous extracts and intraocular pressure of systemic and non-systemic hypertensive subjects. Clin. Exp. Optom. 2015, 98, 138–149. [Google Scholar] [CrossRef] [Green Version]
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef]
- Fuchs, S.; Xiao, H.D.; Hubert, C.; Michaud, A.; Campbell, D.J.; Adams, J.W.; Capecchi, M.R.; Corvol, P.; Bernstein, K.E. Angiotensin-converting enzyme C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension 2008, 51, 267–274. [Google Scholar] [CrossRef]
- Masuyer, G.; Schwager, S.L.; Sturrock, E.D.; Isaac, R.E.; Acharya, K.R. Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Sci. Rep. 2012, 2, 717. [Google Scholar] [CrossRef] [Green Version]
- Martín, N.; Pantoja, C.; Chiang, L.; Bardisa, L.; Araya, C.; Román, R. Hemodynamic effects of a boiling water dialysate of maize silk in normotensive anaesthetized dogs. J. Ethnopharmacol. 1991, 31, 259–262. [Google Scholar] [CrossRef]
- Velazquez, D.V.; Xavier, H.S.; Batista, J.E.; de Castro-Chaves, C. Zea mays L. extracts modify glomerular function and potassium urinary excretion in conscious rats. Phytomedicine 2005, 12, 363–369. [Google Scholar] [CrossRef]
- Fraser, T.B.; Turner, S.W.; Mangos, G.J.; Ludbrook, J.; Whitworth, J.A. Comparison of telemetric and tail-cuff blood pressure monitoring in adrenocorticotrophic hormone-treated rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tao, G.; Liu, P.; Liu, J. Peptide with angiotensin I-converting enzyme inhibitory activity from hydrolyzed corn gluten meal. J. Agric. Food Chem. 2007, 55, 7891–7895. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, S.; Kaneko, T.; Ishikawa, H.; Tanaka, H.; Maruyama, S. Production of bioactive peptides from corn endosperm proteins by some proteases. Ann. N. Y. Acad. Sci. 1995, 750, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Wang, X.; Li, S.; Chen, Z.; Wang, J.; Liu, W. Isolation and identification of a novel peptide from zein with antioxidant and antihypertensive activities. Food Funct. 2015, 6, 3799–3806. [Google Scholar] [CrossRef]
- Ehlers, M.R.; Abrie, J.A.; Sturrock, E.D. C domain-selective inhibition of angiotensin-converting enzyme. J. Renin Angiotensin Aldosterone Syst. 2013, 14, 189–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina, A.S.; Roque, A.C.A. Studies on the molecular recognition between bioactive peptides and angiotensin-converting enzyme. J. Mol. Recognit. 2009, 22, 162–168. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Mathur, D.; Prakash, S.; Anand, P.; Kaur, H.; Agrawal, P.; Mehta, A.; Kumar, R.; Singh, S.; Raghava, G.P. PEPlife: A repository of the half-life of peptides. Sci. Rep. 2016, 6, 36617. [Google Scholar] [CrossRef]
- Santos, R.A.; Krieger, E.M.; Greene, L.J. An improved fluorometric assay of rat serum and plasma converting enzyme. Hypertension 1985, 7, 244–252. [Google Scholar] [CrossRef]
- Costa, M.F.; Carmona, A.K.; Alves, M.F.; Ryan, T.M.; Davies, H.M.; Anderson, G.A.; Slocombe, R.F. Determination of angiotensin I-converting enzyme activity in equine blood: Lack of agreement between methods of analysis. J. Vet. Sci. 2011, 12, 21–25. [Google Scholar] [CrossRef]
- Lo, H.Y.; Ho, T.Y.; Lin, C.; Li, C.C.; Hsiang, C.Y. Momordica charantia and its novel polypeptide regulate glucose homeostasis in mice via binding to insulin receptor. J. Agric. Food Chem. 2013, 61, 2461–2468. [Google Scholar] [CrossRef]
- Peng, Y.C.; Ho, S.P.; Shyu, C.L.; Chang, C.S.; Huang, L.R. Clarithromycin modulates Helicobacter pylori-induced activation of nuclear factor-κB through classical and alternative pathways in gastric epithelial cells. Clin. Exp. Med. 2014, 14, 53–59. [Google Scholar] [CrossRef]
- Access Mascot for free - Protein identification software. Available online: http://www.matrixscience.com (accessed on 1 April 2019).
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar]
- Schneidman-Duhovny, D.; Inbar, Y.; Nussinov, R.; Wolfson, H.J. PatchDock and SymmDock: Servers for rigid and symmetric docking. Nucleic Acids Res. 2005, 33, 363–367. [Google Scholar] [CrossRef]
- PEPstrMOD: Peptide Tertiary Structure Prediction with Natural, Non-Natural and Modified Residues. Available online: http://osddlinux.osdd.net/raghava/pepstrmod/ multiseq.php (accessed on 1 April 2019).
- UCSF Chimera. Available online: https://www.cgl.ucsf.edu/chimera/ (accessed on 1 April 2019).
- LIGPLOT. Available online: https://www.ebi.ac.uk/thornton-srv/software/ LIGPLOT/ (accessed on 1 April 2019).
- Wang, C.; Zhang, T.; Liu, J.; Lu, S.; Zhang, C.; Wang, E.; Wang, Z.; Zhang, Y.; Liu, J. Subchronic toxicity study of corn silk with rats. J. Ethnopharmacol. 2011, 137, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.Z.; Zhang, S.Y.; Zhou, H.L. Toxicological evaluation of the flavonoid-rich extract from Maydis stigma: Subchronic toxicity and genotoxicity studies in mice. J. Ethnopharmacol. 2016, 192, 161–169. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Spot Number | Protein Name | Accession Number | Length | Molecular Weight (Da) | Score 1 | PSM 2 | Coverage (%) 3 |
|---|---|---|---|---|---|---|---|
| 1 | Acidic endochitinase | ONM51744 | 308 | 33,748 | 274 | 17 | 31.82 |
| 2 | Lipoxygenase | NP_001105003 | 873 | 98,164 | 206 | 32 | 4.81 |
| 3 | Heat shock 70 kDa protein 4 | ACG43420 | 848 | 93,569 | 166 | 7 | 5.90 |
| 4 | Ascorbate peroxidase | ACO90192 | 250 | 27,597 | 78 | 8 | 8.00 |
| 5 | Adenosylhomocysteinase | NP_001148534 | 485 | 53,248 | 349 | 22 | 22.06 |
| 6 | APx3-Peroxisomal ascorbate peroxidase | NP_001148710 | 290 | 32,072 | 67 | 7 | 2.76 |
| 7 | Uncharacterized LOC100216603 | NP_001336786 | 129 | 14,353 | 45 | 3 | 15.50 |
| 8 | 60S Ribosomal protein L37a-2 | ONM18155 | 194 | 21,777 | 152 | 5 | 12.37 |
| 9 | NADP-dependent malic enzyme | ACX50497 | 608 | 67,164 | 154 | 15 | 9.87 |
| 10 | Trypsin inhibitor precursor | NP_001152433 | 175 | 19,060 | 120 | 6 | 17.14 |
| 11 | Cytochrome P450 CYP74A19 | ACG28578 | 483 | 53,105 | 96 | 11 | 13.67 |
| Peptide 1 | Amino Acid Sequence | Molecular Weight (Da) | Ion Score 2 | Mass Error (ppm) | Docking Score 3 | Area 4 |
|---|---|---|---|---|---|---|
| CSBp1 | CGFPPAGYLRR | 1293 | 24 | −1.470 | 10,718 | 1569.4 |
| CSBp2 | DAPWWPK | 898 | 31 | 0.557 | 7890 | 1018.7 |
| CSBp3 | DLASFPFR | 951 | 37 | 0.420 | 9928 | 1215.1 |
| CSBp4 | NCAPLMLR | 989 | 27 | 0.616 | 9788 | 1429.9 |
| CSBp5 | SKFDNLYGCR | 1258 | 65 | 0.715 | 10,872 | 1498.7 |
| CSBp6 | NCAPIMLR | 989 | 27 | −6.670 | 8938 | 1111.3 |
| CSBp7 | AMPTFFLIK | 1066 | 11 | 0.554 | 10,596 | 1438.5 |
| CSBp8 | YFCEFCGK | 1109 | 36 | 0.811 | 8318 | 1203.8 |
| CSBp9 | GLIYPPFSNIR | 1275 | 56 | 0.784 | 9930 | 1630.6 |
| CSBp10 | EPFIRPPR | 1010 | 31 | 1.880 | 9828 | 1479.5 |
| CSBp11 | MNVPPGPFMAR | 1215 | 58 | −3.537 | 10,428 | 1380.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-C.; Lee, Y.-C.; Lo, H.-Y.; Huang, Y.-W.; Hsiang, C.-Y.; Ho, T.-Y. Antihypertensive Effects of Corn Silk Extract and Its Novel Bioactive Constituent in Spontaneously Hypertensive Rats: The Involvement of Angiotensin-Converting Enzyme Inhibition. Molecules 2019, 24, 1886. https://doi.org/10.3390/molecules24101886
Li C-C, Lee Y-C, Lo H-Y, Huang Y-W, Hsiang C-Y, Ho T-Y. Antihypertensive Effects of Corn Silk Extract and Its Novel Bioactive Constituent in Spontaneously Hypertensive Rats: The Involvement of Angiotensin-Converting Enzyme Inhibition. Molecules. 2019; 24(10):1886. https://doi.org/10.3390/molecules24101886
Chicago/Turabian StyleLi, Chia-Cheng, Yu-Chen Lee, Hsin-Yi Lo, Yu-Wen Huang, Chien-Yun Hsiang, and Tin-Yun Ho. 2019. "Antihypertensive Effects of Corn Silk Extract and Its Novel Bioactive Constituent in Spontaneously Hypertensive Rats: The Involvement of Angiotensin-Converting Enzyme Inhibition" Molecules 24, no. 10: 1886. https://doi.org/10.3390/molecules24101886