Synthesis of Thymidine Phosphorylase Inhibitor Based on Quinoxaline Derivatives and Their Molecular Docking Study
Abstract
:1. Introduction:
2. Results and Discussion
2.1. Chemistry
2.2. In vitro Thymidine Phosphorylase Inhibitory Activity
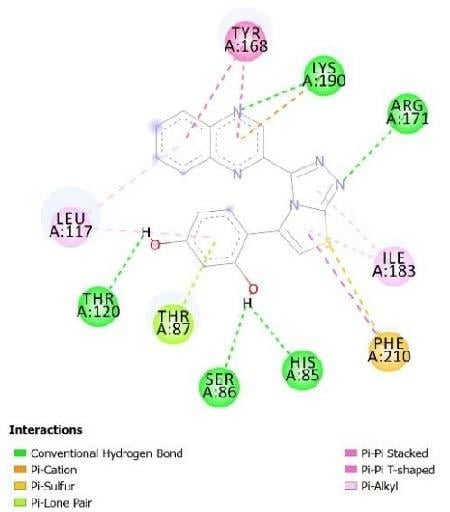
2.3. Molecular Docking
3. Experimental Section
3.1. General Methods
3.1.1. Thymidine Phosphorylase Assay
3.1.2. Calculations
3.1.3. Synthesis of Quinoxaline Thiosemicarbazone (II)
3.1.4. Synthesis of 5-(quinoxalin-3-yl)-4H-1,2,4-triazole-3-thiol (III)
3.2. General Procedure for Synthesis of Quinoxaline Derivatives (1–25)
3.2.1. 5-(2-flourophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.2. 5-(3-flourophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.3. 5-(4-flourophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.4. 5-(2-chlorophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.5. 5-(3-chlorophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.6. 5-(4-chlorophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.7. 5-(2-nitrophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.8. 5-(4-nitrophenyl)-3(Quinoxalin-2yl)thiazolo[2,3-c][1,2,4]triazole
3.2.9. 3-(quinoxalin-2-yl)-5-o-tolylthiazolo[2,3-c][1,2,4]triazole
3.2.10. 3-(quinoxalin-2-yl)-5-m-tolylthiazolo[2,3-c][1,2,4]triazole
3.2.11. 3-(quinoxalin-2-yl)-5-p-tolylthiazolo[2,3-c][1,2,4]triazole
3.2.12. 3-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)phenol
3.2.13. 4-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)phenol
3.2.14. 4-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)benzene-1,3-diol
3.2.15. 2-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)benzene-1,4-diol
3.2.16. 4-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)benzene-1,2-diol
3.2.17. 5-methoxy-2-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)phenol
3.2.18. 2-methoxy-5-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)phenol
3.2.19. 5-(3-methoxyphenyl)-3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazole
3.2.20. 5-(4-methoxyphenyl)-3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazole
3.2.21. 4-methoxy-2-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)phenol
3.2.22. 5-(pyridin-3-yl)-3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazole
3.2.23. 5-(pyridin-4-yl)-3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazole
3.2.24. 2-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)phenol
3.2.25. 3-(3-(quinoxalin-2-yl)thiazolo[2,3-c][1,2,4]triazol-5-yl)benzene-1,2-diol
3.3. Molecular Docking Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esteban, G.A.; Balzarini, J.; Esnouf, R.; Clercq, E.D.; Camarasa, M.J.; Pérez-Pérez, M.J. Design, Synthesis, and Enzymatic Evaluation of Multisubstrate Analogue Inhibitors of Escherichia coli Thymidine Phosphorylase. J. Med. Chem. 2000, 43, 971–983. [Google Scholar] [CrossRef]
- Friedkin, M.; Roberts, D. Der Stoffwechsel der Purine und Pyrimidine. J. Biol. Chem. 1954, 207, 245–256. [Google Scholar] [PubMed]
- Krenitsky, T.A.; Barclay, M.; Jacquez, J.A. Specificity of mouse uridine phosphorylase. J. Biol. Chem. 1964, 239, 805–812. [Google Scholar] [PubMed]
- Iltzsch, M.H.; el-Kouni, M.H.; Cha, S. Kinetic studies of thymidine phosphorylase from mouse liver. Biochemistry 1985, 24, 6799–6807. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Bicknell, R. Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem. J. 1998, 334, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.; Yoshimura, A.; Sumizawa, T.; Haraguchi, M.; Akiyama, S.I.; Fukui, K.; Ishizawa, M.; Yamada, Y. Angiogenic factor. Nature 1992, 356, 668. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, M.; Miyadera, K.; Uemura, K.; Sumizawa, T.; Furukawa, T.; Yamada, K.; Akiyama, S.I.; Yamada, Y. Angiogenic activity of enzymes. Nature 1994, 368, 198. [Google Scholar] [CrossRef] [PubMed]
- Takao, S.; Akiyama, S.; Nakajo, A.; Yoh, H.; Kitazono, M.; Natsugoe, S.; Miyadera, K.; Fukushima, M.; Yamada, Y.; Aikou, T. Suppression of metastasis by thymidine phosphorylase inhibitor. Cancer Res. 2000, 60, 5345–5348. [Google Scholar]
- Bronckaers, A.; Gago, F.; Balzarini, J.; Liekens, S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med. Res. Rev. 2009, 29, 903–953. [Google Scholar] [CrossRef]
- Akiyama, S.I.; Furukawa, T.; Sumizawa, T.; Takebayashi, Y.; Nakajima, Y.; Shimaoka, S.; Haraguchi, M. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004, 95, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Sumizawa, T.; Furukawa, T.; Haraguchi, M.; Yoshimura, A.; Takeyasu, A.; Ishizawa, M.; Yamada, Y.; Akiyama, S.I. Thymidine phosphorylase activity associated with platelet-derived endothelial cell growth factor. J. Biochem. 1993, 114, 9–14. [Google Scholar] [CrossRef]
- Usuki, K.; Saras, J.; Waltenberger, J.; Miyazono, K.; Pierce, G.; Thomason, A.; Heldin, C.H. Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity. Biochem. Biophys. Res. Commun. 1992, 184, 1311–1316. [Google Scholar] [CrossRef]
- Abbas, Y.; Mansha, M.; Ullah, N. The first total synthesis of potent antitumoral(±)- mafaicheenamine A, unnatural 6-fluoromafaicheenamine A and expedient synthesis of clausine E. RSC Adv. 2016, 6, 26104–26110. [Google Scholar] [CrossRef]
- Matsushita, S.; Nitanda, T.; Furukawa, T.; Sumizawa, T.; Tani, A.; Nishimoto, K.; Akiba, S.; Miyadera, K.; Fukushima, M.; Yamada, Y.; et al. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer Res. 1999, 59, 1911–1916. [Google Scholar]
- Focher, F.; Spadari, S. Thymidine phosphorylase: A two-face Janus in anticancer chemotherapy. Curr. Cancer Drug Targets 2001, 1, 141–153. [Google Scholar] [CrossRef]
- Miyadera, K.; Emura, T.; Suzuki, N.; Akiyama, S.; Fukushima, M.; Yamada, Y. Novel functional antitumor nucleoside TAS-102, combined from of F3Rhd and its modulator (2): Inhibitory effect of TPI on tumor-derived angiogenesis and metasis. Proc. Am. Assoc. Cancer Res. 1998, 39, 609. [Google Scholar]
- Pomeisl, K.; Votruba, I.; Holy, A.; Pohl, R. Nucleosides, Syntheses of pyrimidine acyclic nucleoside phosphonates as potent inhibitors of thymidine phosphorylase (PD-ECGF) from SD-lymphoma. Nucleotides Nucleic Acids 2007, 26, 1025–1028. [Google Scholar] [CrossRef]
- Gbaj, A.; Edwards, P.N.; Reigan, P.; Freeman, S.; Jaffar, M.; Douglas, K.T. Thymidine phosphorylase from Escherichia coli: Tight-binding inhibitors as enzyme active-site titrants. J. Enzym. Inhib. Med. Chem. 2006, 21, 69–73. [Google Scholar] [CrossRef]
- Nencka, R.; Votruba, I.; Hrebabecky, H.; Jansa, P.; Tloustova, E.; Horska, K.; Masojidkova, M.; Holy, A. Discovery of 5-substituted-6-chlorouracils as efficient inhibitors of human thymidine phosphorylase. J. Med. Chem. 2007, 50, 6016–6023. [Google Scholar] [CrossRef]
- McNally, V.A.; Rajabi, M.; Gbaj, A.; Stratford, I.J.; Edwards, P.N.; Douglas, K.T.; Bryce, R.A.; Jaffar, M.; Freeman, S. Design, synthesis and enzymatic evaluation of 6-bridged imidazolyluracil derivatives as inhibitors of human thymidine phosphorylase. J. Pharm. Pharmacol. 2007, 59, 537–547. [Google Scholar] [CrossRef]
- Liekens, S.; Balzarini, J.; De Hernández, A.I.; Clercq, E.; Priego, E.M.; Camarasa, M.J.; Pérez-Pérez, M.J. Thymidine Phosphorylase is Noncompetitively Inhibited by 5′-O-Trityl-Inosine (KIN59) and Related Compounds. Nucleosides Nucleotides Nucleic Acids 2006, 25, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Casanova, E.; Hernandez, A.I.; Priego, E.M.; Liekens, S.; Camarasa, M.J.; Balzarini, J.; Pérez-Pérez, M.J. 5′-O-tritylinosine and analogues as allosteric inhibitors of human thymidine phosphorylase. J. Med. Chem. 2006, 49, 5562–5570. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, M.J.; Priego, E.M.; Hernandez, A.I.; Camarasa, M.J.; Balzarini, J.; Liekens, S. Thymidine phosphorylase inhibitors: Recent developments and potential therapeutic applications. Mini Rev. Med. Chem. 2005, 5, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Bera, H.; Chui, W.K. Synthesis of pyrazolo[1,5-a][1,3,5]triazine derivatives as inhibitors of thymidine phosphorylase. Eur. J. Med. Chem. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Kazuno, H.; Sato, T.; Suzuki, N.; Emura, T.; Wierzba, K.; Yamashita, J.I.; Tada, Y.; Yamada, Y.; Fukushima, M.; et al. Synthesis and evaluation of 6-methylene-bridged uracil derivatives. Part 2: Optimization of inhibitors of human thymidine phosphorylase and their selectivity with uridine phosphorylase. Bioorg. Med. Chem. Lett. 2004, 12, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Gamboa, A.E.; Esnouf, R.; Liekens, S.; Neyts, J.; De Clercq, E.; Camarasa, M.J.; Pérez-Pérez, M.J. 7-Deazaxanthine, a novel prototype inhibitor of thymidine phosphorylase, FEBS Lett. 1998, 438, 91–95. FEBS Lett. 1998, 438, 91–95. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Karcz, D.; Pustuła, K.; Makowski, M.; Górecki, A.; Kluczyk, D.; Karpińska, M.M.; Niewiadomy, A.; Gagoś, M. Spectroscopic and theoretical studies of fluorescence effects in bio-active: 4-(5-(methyl-1,3,4-thiadiazol-2-yl)) benzene-1,3-diol and 4-(5-(methylamino-1, 3, 4-thiadiazol-2-yl)) benzene-1, 3-diol compounds: Effect of molecular aggregation and amino group position. J. Fluoresc. 2018, 201, 44–56. [Google Scholar]
- Tiwari, S.V.; Siddiqui, S.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.P. Microwave-assisted facile synthesis, anticancer evaluation and docking study of N-((5-(substituted methylene amino)-1, 3, 4-thiadiazol-2-yl) methyl) benzamide derivatives. Molecules 2017, 15, 995. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Deivedi, S.K.; Hashim, S.R.; Singhal, R.G. Synthesis and antimicrobial activity of some new quinoxaline derivatives. Pharmaceuticals 2010, 3, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.L.P.; Suchakar, D.; Vashu, K.; Rao, C.V. Synthesis and biological activity of 8-chloro-[1,2,4]triazolo[4,3-a]quinoxalines. J. Chem. Pharm. Res. 2010, 2, 497–504. [Google Scholar]
- Patidar, A.J.M.; Mobiya, A.; Selvam, G. Internat. Exploring potential of quinoxaline moiety. J. Pharm. Tech. Res. 2011, 3, 386–392. [Google Scholar]
- Vieira, M.; Pinheiro, C.; Fernandes, R.; Noronha, J.P.; Prudêncio, C. Antimicrobial activity of quinoxaline 1,4-dioxide with 2-and 3-substituted derivatives. Microbiol. Res. 2014, 169, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Azam, A. Synthesis, characterization and antiamoebic activity of 1-(thiazolo [4,5-b] quinoxaline-2-yl)-3-phenyl-2-pyrazoline derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Abu-Hashem, A.A.; Gouda, M.A.; Badria, F.A. Synthesis of some new pyrimido [2′, 1′: 2,3] thiazolo [4,5-b] quinoxaline derivatives as anti-inflammatory and analgesic agents. Euro. J. Med. Chem. 2010, 45, 1976–1981. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and anticancer activity evaluation of new 2-alkylcarbonyl and 2-benxoyl-3-trifluoromethyl-quinoxaline 1,4-do-N-oxide derivatives. Bioorg. Med. Chem. 2004, 12, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Husain, A. Madhesia, Recent advances in pharmacological activities of quinoxaline derivates. J. Pharm. Res. 2011, 4, 924–929. [Google Scholar]
- Deepika, Y.; Surendra, P.; Sachin, N.K.; Shewta, S. Biological activity of quinoxaline derivatives. Int. J. Curr. Pharm. Rev. Res. 2011, 1, 33–46. [Google Scholar]
- Chung, H.J.; Jung, O.J.; Chae, M.J.; Hong, S.Y.; Chung, K.H.; Lee, S.K.; Ryu, C.K. Synthesis and biological evaluation of quinoxaline-5, 8-diones that inhibit vascular smooth muscle cell proliferation. Bioorg. Med. Chem. Lett. 2005, 15, 3380–3384. [Google Scholar] [CrossRef] [PubMed]
- Noreen, T.; Taha, M.; Imran, S.; Chigurpati, S.; Rahim, F.; Selvaraj, M.; Ismail, N.H.; Mohammad, J.I.; Ullah, H.; Javid, M.T.; et al. Synthesis of Alpha Amylase Inhibitors Based on Privileged Indole Scaffold. Bioorg. Chem. 2017, 72, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ali, M.; Ullah, S.; Rashid, U.; Ullah, H.; Taha, M.; Javed, M.T.; Rehman, W.; Abid, O.U.R.; Khan, A.A.; et al. Development of bis-Thiobarbiturates as Successful Urease Inhibitors and their Molecular Modeling Studies. Chin. Chem. Lett. 2016, 27, 693–697. [Google Scholar] [CrossRef]
- Taha, M.; Sultan, S.; Nuzar, H.A.; Rahim, F.; Imran, S.; Ismail, N.H.; Naz, H.; Ullah, H. Synthesis and biological evaluation of novel N-arylidenequinoline-3-carbohydrazides as potent β-glucuronidase inhibitors. Bioorg. Med. Chem. 2016, 24, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Rashid, U.; Rahim, F.; Taha, M.; Arshad, M.; Ullah, H.; Mahmood, T.; Ali, M. Synthesis of 2-Acylated and Sulfonated 4-hydroxycoumarins: In vitro Urease Inhibition and Molecular Docking Studies. Bioorg. Chem. 2016, 66, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Imran, S.; Ismail, N.H.; Selvaraj, M.; Rahim, F.; Chigurupati, S.; Ullah, H.; Khan, F.; Salar, U.; Javid, M.T.; et al. Biology-oriented drug synthesis (BIODS) of 2-(2-methyl-5-nitro-1Himidazol-1-yl)ethyl aryl ether derivatives, in vitro α-amylase inhibitory activity and in silico studies. Bioorg. Chem. 2017, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, K.; Ullah, H.; Wadood, A.; Taha, M.; Rehman, A.; din, I.U.; Ashraf, M.; Shaukat, A.; Rehman, W.; et al. Triazinoindole analogs as potent inhibitors of α-glucosidase: Synthesis, biological evaluation and molecular docking studies. Bioorg. Chem. 2015, 58, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Imran, S.; Rahim, F.; Wadood, A.; Khan, H.; Ullah, H.; Salar, U.; Khan, K.M. Synthesis, β-Glucuronidase Inhibition and Molecular Docking Studies of Hybrid Bisindole-Thiosemicarbazides Analogs. Bioorg. Chem. 2016, 68, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Javid, M.T.; Wadood, A.; Taha, M.; Ashraf, M.; Shaukat, A.; Junaid, M.; Hussain, S.; Rehman, W.; et al. Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of α-glucosidase. Bioorg. Chem. 2015, 62, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Javid, M.T.; Ullah, H.; Wadood, A.; Taha, M.; Ashraf, M.; Aine, Q.U.; Khan, M.A.; Khan, F.; Mirza, S.; et al. Synthesis, Molecular Docking, Acetylcholinesterase and Butyrylcholinesterase Inhibitory Potential of Thiazole Analogs as New Inhibitors for Alzheimer Disease. Bioorg. Chem. 2015, 62, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Malik, F.; Ullah, H.; Wadood, A.; Khan, F.; Javid, M.T.; Taha, M.; Rehman, W.; Rehman, A.U.; Khan, K.M. Isatin based Schiff bases as inhibitors of α-glucosidase: Synthesis, characterization, in vitro evaluation and molecular docking studies. Bioorg. Chem. 2015, 60, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rahim, F.; Ullah, H.; Taha, M.; Wadood, A.; Javid, M.T.; Rehman, W.; Nawaz, M.; Ashraf, M.; Ali, M.; Sajid, M.; et al. Synthesis and in vitro Acetylcholinesterase and Butyrylcholinesterase Inhibitory Potential of Hydrazide based Schiff Bases. Bioorg. Chem. 2016, 68, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Uddin, I.; Taha, M.; Rahim, F.; Wadood, A. Synthesis and molecular docking study of piperazine derivatives as potent inhibitor of thymidine phosphorylase. Bioorg. Chem. 2018, 78, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Rashid, U.; Imran, S.; Ali, M. Rational design of bis-indolylmethane-oxadiazole hybrids as inhibitors of thymidine phosphorylase. Bioorg. Med. Chem. 2018, 26, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Barbuceanu, S.F.; Almajan, G.L.; Saramet, I.; Draghici, C.; Tarcomnicu, A.I.; Bancescu, G. Synthesis, characterization and evaluation of antibacterial activity of some thiazolo [3,2-b][1,2,4] triazole incorporating diphenylsulfone moieties. Eur. J. Med. Chem. 2009, 44, 4752–4757. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thymidine Phosphorylase from E. coli with 3′-Azido-2′-Fluoro-Dideoxyuridine. Available online: http://www.rcsb.org/structure/4EAD (accessed on 27 March 2013).
Sample Availability: Samples of the compounds are available from the authors. |








| S.No. | R | IC50 (µM ± SEM a) | S.No. | R | IC50 (mM ± SEM a) |
|---|---|---|---|---|---|
| 1 |  | 13.60 ± 0.4 | 14 |  | 13.20 ± 0.40 |
| 2 |  | 26.10 ± 0.70 | 15 |  | 15.20 ± 0.50 |
| 3 |  | 18.10 ± 0.50 | 16 |  | 3.50 ± 0.20 |
| 4 |  | 27.40 ± 0.60 | 17 |  | 24.20 ± 0.70 |
| 5 |  | 33.40 ± 0.80 | 18 |  | 16.90 ± 0.60 |
| 6 |  | 24.40 ± 0.60 | 19 |  | N. A. |
| 7 |  | 34.70 ± 0.80 | 20 |  | N. A. |
| 8 |  | 47.50 ± 0.90 | 21 |  | 26.20 ± 0.50 |
| 9 |  | 56.40 ± 1.20 | 22 |  | N. A. |
| 10 |  | N. A. | 23 |  | N. A. |
| 11 |  | N. A. | 24 |  | 13.10 ± 0.30 |
| 12 |  | 33.20 ± 0.75 | 25 |  | 3.20 ± 0.10 |
| 13 |  | 18.30 ± 0.55 | - | - | - |
| 7-Deazaxanthine (7DX) | 38.68 ± 4.42 µM | ||||
| No. of Compound | Free Binding Energy (kcal/mol) | H-Bonds (HBs) | Number of Closest Residues to the Docked Ligand in The Active Site | IC50 ± SEM |
|---|---|---|---|---|
| 14 | −7.71 | 5 | 10 | 13.20 ± 0.40 |
| 15 | −7.61 | 3 | 8 | 15.20 ± 050 |
| 16 | −8.05 | 3 | 8 | 3.50 ± 0.20 |
| 25 | −8.25 | 4 | 8 | 3.20 ± 0.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almandil, N.B.; Taha, M.; Farooq, R.K.; Alhibshi, A.; Ibrahim, M.; Anouar, E.H.; Gollapalli, M.; Rahim, F.; Nawaz, M.; Shah, S.A.A.; et al. Synthesis of Thymidine Phosphorylase Inhibitor Based on Quinoxaline Derivatives and Their Molecular Docking Study. Molecules 2019, 24, 1002. https://doi.org/10.3390/molecules24061002
Almandil NB, Taha M, Farooq RK, Alhibshi A, Ibrahim M, Anouar EH, Gollapalli M, Rahim F, Nawaz M, Shah SAA, et al. Synthesis of Thymidine Phosphorylase Inhibitor Based on Quinoxaline Derivatives and Their Molecular Docking Study. Molecules. 2019; 24(6):1002. https://doi.org/10.3390/molecules24061002
Chicago/Turabian StyleAlmandil, Noor Barak, Muhammad Taha, Rai Khalid Farooq, Amani Alhibshi, Mohamed Ibrahim, El Hassane Anouar, Mohammed Gollapalli, Fazal Rahim, Muhammad Nawaz, Syed Adnan Ali Shah, and et al. 2019. "Synthesis of Thymidine Phosphorylase Inhibitor Based on Quinoxaline Derivatives and Their Molecular Docking Study" Molecules 24, no. 6: 1002. https://doi.org/10.3390/molecules24061002