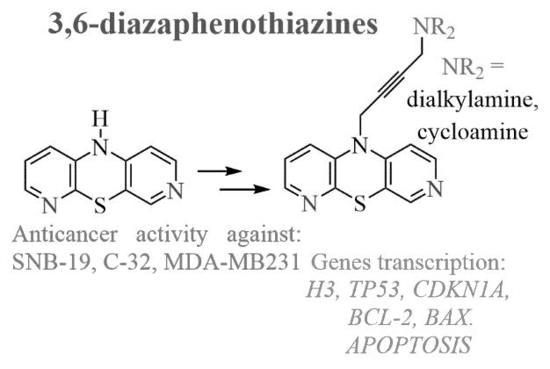
Synthesis, Anticancer Activity, and Apoptosis Induction of Novel 3,6-Diazaphenothiazines †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anticancer Activity
2.3. Apoptosis Assay
3. Materials and Methods
3.1. Chemistry
General Procedure for Synthesis of Compounds (3–9)
3.2. Anticancer Effects In Vitro
3.2.1. Cell Culture
3.2.2. Cell Proliferation and Viability
3.2.3. RT-qPCR Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.; Siegel, R.; Jemal, A. Global Cancer Facts and Figures, 3rd ed.; American Cancer Society Atlanta: Atlanta, GA, USA, 2015; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-3rd-edition.pdf (accessed on 30 November 2018).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer, J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, M.D.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ren, J.-S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2013, 32, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Viveiros, M. Thioridazine: A non-antibiotic drug highly effective, in combination with first line anti-tuberculosis drugs, against any form of antibiotic resistance of mycobacterium tuberculosis due to its multi-mechanisms of action. Antibiotics 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Huang, D.; Qin, L.; Zheng, Z.; Hua, L.; Wang, G.; Huang, J.; Huang, H. Targeting lung cancer stem cells with antipsychological drug thioridazine. Bio. Med. Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. J. Heterocycl. Chem. 2009, 46, 355–391. [Google Scholar] [CrossRef]
- Motohashi, N.; Kawase, M.; Satoh, K.; Sakagami, H. Cytotoxic potential of phenothiazines. Curr. Drug Targets 2006, 7, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C. Phenothiazine: The parent molecule. Curr. Drug Targets 2006, 7, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Mosnaim, A.D.; Ranade, V.V.; Wolf, M.E. Phenothiazine molecule provides the basic chemical structure for various classes of pharmacotherapeutic agents. Am. J. Therapeut. 2006, 13, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Dastridara, S.G.; Shirataki, Y.; Motohashi, N. Antibacterial activity of artificial phenothiazines and isoflavones from plants. Bioact. Heterocycles 2008, 15, 67–132. [Google Scholar]
- Aaron, J.J.; Gaye Seye, M.D.; Trajkovska, S.; Motohashi, N. Bioactive phenothiazines and benzo[a]phenothiazines: Spectroscopic studies and biological and biomedical properties and applications. Bioact. Heterocycles 2009, 153–231. [Google Scholar] [CrossRef]
- Sadandam, Y.S.; Shetty, M.M.; Bhaskar Rao, A. 10H-Phenothiazines: A new class of enzyme inhibitorsfor inflammatory diseases. Eur. J. Med. Chem. 2009, 44, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Sudeshna, G.; Parimal, K. Multiple non-psychiatric effect of phenothiazines: A review. Eur. J. Pharmacol. 2010, 648, 6–14. [Google Scholar] [CrossRef]
- Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Recent progress in biological activities of synthesized phenothiazines. Eur. J. Med. Chem. 2011, 46, 3179–3189. [Google Scholar] [CrossRef]
- Jaszczyszyn, A.; Gąsiorowski, K.; Świątek, P.; Malinka, W.; Cieślik-Boczula, K.; Petrus, J.; Czarnik-Matusewicz, B. Chemical structure of phenothiazines and their biological activity. Pharmacol. Rep. 2012, 64, 16–23. [Google Scholar] [CrossRef]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M.; Zaczyńska, E. Azaphenothiazines a promising phenothiazine derivatives. An insight into nomenclature, synthesis, structure elucidation and biological properties. Eur. J. Med. Chem. 2017, 138, 774–806. [Google Scholar] [CrossRef]
- Viveiros, M.; Martins, M.; Couto, I.; Kristiansen, J.E.; Molnar, J.; Amaral, L. The In vitro activity of phenothiazines against mycobacterium avium: Potential of thioridazine for therapy of the co-infected AIDS patient. In Vivo 2005, 19, 733–736. [Google Scholar]
- González-Muñoz, G.C.; Arce, M.P.; López, B.; Pérez, C.; Romero, A.; del Barrio, L.; Martín-de-Saavedra, M.D.; Egea, J.; León, R.; Villarroya, M.; et al. N-Acylamino-phenothiazines: Neuroprotective agents displaying multifunctional activities for a potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2011, 46, 2224–2235. [Google Scholar] [CrossRef]
- González-Muñoz, G.C.; Arce, M.P.; López, B.; Pérez, C.; Villarroya, M.; López, M.G.; García, A.G.; Conde, S.; Rodríguez-Franco, M.I. Old phenothiazine and dibenzothiadiazepine derivatives for tomorrow’s neuroprotective therapies against neurodegenerative diseases. Eur. J. Med. Chem. 2010, 45, 6152–6158. [Google Scholar] [CrossRef]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef]
- Kaur, P.; Chu, J.J.H. Chikungunya virus: An update on antiviral development and challenges. Drug Discov. Today 2013, 18, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Pluta, K.; Jeleń, M.; Morak-Młodawska, B.; Zimecki, M.; Artym, J.; Kocięba, M. Anticancer activity of newly synthesized azaphenothiazines in NCI’s anticancer screening. Pharmacol. Rep. 2010, 62, 319–332. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Matralis, A.N.; Kourounakis, A.P. Antioxidant activity of newly synthesized 2,7-diazaphenothiazines. Arch. Pharm. Chem. Life Sci. 2010, 343, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Artym, J.; Kocięba, M.; Pluta, K.; Morak-Młodawska, B.; Jeleń, M. Immunosupressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell Mol. Biol. Lett. 2009, 14, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Zimecki, M.; Jeleń, M.; Artym, J.; Kocięba, M. Synthesis and selected immunological properties of 10-substituted 1,8-diazaphenothiazines. Med. Chem. Res. 2015, 24, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Jeleń, M. Estimation of the lipophilicity of new anticancer and immunosuppressive 1,8-diazaphenothiazine derivatives. J. Chromatogr. Sci. 2015, 53, 462–466. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M. Synthesis, spectroscopic characterization, and anticancer activity of new 10-substituted 1,6-diazaphenothiazines. Med. Chem. Res. 2016, 25, 2425–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artym, J.; Kochanowska, E.; Kocięba, M.; Zaczyńska, E.; Zimecki, M.; Jeleń, M.; Morak-Młodawska, B.; Pluta, K. Selected azaphenothiazines inhibit delayd type hiper-sensivity and carrageenan reaction in mice. Int. Immunopharmacol. 2016, 40, 265–268. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef]
- Bisi, A.; Meli, M.; Gobbi, S.; Rampa, A.; Tolomeo, M.; Dusonchet, L. Multidrug resistance reverting activity and antitumor profile of new phenothiazine derivatives. Bioorg. Med. Chem. 2008, 16, 6474–6482. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis and anticancer and lipophilic properties of 10-dialkylaminobutynyl derivatives of 1,8- and 2,7-diazaphenothiazines. J. Enzyme Inhib. Med. Chem. 2016, 31, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Suwińska, K.; Jeleń, M.; Kuśmierz, D. 3,6-Diazaphenothiazines as potential lead molecules—Synthesis, characterization and anticancer activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Wenzhi, Z.; Okechukwu, N.P.; Morak-Młodawska, B.; Pluta, K.; Jeleń, M.; Md Akim, A.; Ang, K.-P.; Ooi, K.K. 10H-3,6-Diazaphenothiazines induce G2/M phase cell cycle arrest, caspase-dependent apoptosis and inhibits cell invasion of A2780 ovarian carcinoma cells through regulation on NF-κB and [BIRC6-XIAP] complexes. Drug Des. Develop. Ther. 2017, 11, 3045–3063. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Lowe, S.W. The p53-BCL-2 connection. Cell Death Differ. 2006, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Andrais, B.; Scott, A.; Murray, D. New insights into p53 signaling and cancer cell response to DNA damage: Implications for cancer therapy. J. Biomed. Biotech. 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; Korsmeyer, S.J. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 2003, 304, 437–444. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–605. [Google Scholar] [CrossRef]
- Zhu, A.K.; Zhou, H.; Xia, J.Z.; Jin, H.C.; Wang, K.; Yan, J.; Zuo, J.B.; Zhu, X.; Shan, T. Ziyuglycoside II-induced apoptosis in human gastric carcinoma BGC-823 cells by regulating Bax/Bcl-2 expression and activating caspase-3 pathway. Braz. J. Med. Biol. Res. 2013, 46, 670–675. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |

| No | Anticancer Activity IC50 (μg/mL) | ||
|---|---|---|---|
| SNB-19 | C-32 | MDA-MB231 | |
| 2 | 45.1 [23] | 32.9 [23] | 37.9 a [23] |
| 3 | 0.11 ± 0.1 | 1.53 ± 0.6 | 2.81 ± 0.8 |
| 4 | 0.11 ± 0.1 | 0.54 ± 0.2 | 6.32 ± 1.2 |
| 5 | >50 | >50 | >50 |
| 6 | >50 | >50 | >50 |
| 7 | >50 | >50 | >50 |
| 8 | 2.43 ± 1.2 | >50 | 7.64 ± 2.2 |
| 9 | 0.45 ± 0.1 | 4.57 ± 1.1 | 0.74 ± 0.3 |
| Cisplatin | 1.12 ± 0.4 | 3.96 ± 0.2 | 7.75 ± 1.6 |
| Gene | Compound | SNB-19 | C-32 | MDA-MB231 |
|---|---|---|---|---|
| Number of mRNA Copies/μg Total RNA | ||||
| H3 | control | 17909 ± 1812 | 995904 ± 181187 | 183104 ± 32057 |
| 4 | 9006 ± 825 | 364206 ± 45530 | 48424 ± 6183 | |
| TP53 | control | 534284 ± 53484 | 682740 ± 84733 | 181536 ± 4213 |
| 4 | 522817 ± 81864 | 349227 ± 33057 | 170163 ± 13984 | |
| CDKN1A | control | 242104 ± 131105 | 1752117 ± 374944 | 33965 ± 2504 |
| 4 | 324905 ± 10763 | 918317 ± 25533 | 49764 ± 2445 | |
| BCL-2 | control | 15316 ± 1085 | 68431 ± 12162 | 121242 ± 13880 |
| 4 | 9392 ± 2312 | 32278 ± 1323 | 109429 ± 10990 | |
| BAX | control | 918016 ± 47035 | 2431793 ± 574892 | 154192 ± 11428 |
| 4 | 1061056 ± 165795 | 1053715 ± 155279 | 143037 ± 18827 | |
| BAX/BCL-2 | control | 55.9 | 35.5 | 1.27 |
| 4 | 113 | 32.6 | 1.31 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morak-Młodawska, B.; Pluta, K.; Latocha, M.; Jeleń, M.; Kuśmierz, D. Synthesis, Anticancer Activity, and Apoptosis Induction of Novel 3,6-Diazaphenothiazines. Molecules 2019, 24, 267. https://doi.org/10.3390/molecules24020267
Morak-Młodawska B, Pluta K, Latocha M, Jeleń M, Kuśmierz D. Synthesis, Anticancer Activity, and Apoptosis Induction of Novel 3,6-Diazaphenothiazines. Molecules. 2019; 24(2):267. https://doi.org/10.3390/molecules24020267
Chicago/Turabian StyleMorak-Młodawska, Beata, Krystian Pluta, Małgorzata Latocha, Małgorzata Jeleń, and Dariusz Kuśmierz. 2019. "Synthesis, Anticancer Activity, and Apoptosis Induction of Novel 3,6-Diazaphenothiazines" Molecules 24, no. 2: 267. https://doi.org/10.3390/molecules24020267