Antileishmanial Activity of Dimeric Flavonoids Isolated from Arrabidaea brachypoda
Abstract
:1. Introduction
2. Results
2.1. In Vitro Activity Against Leishmania Promastigotes and Cytotoxicity to Mammalian Cells
2.2. Activity Against Amastigotes of L. amazonensis
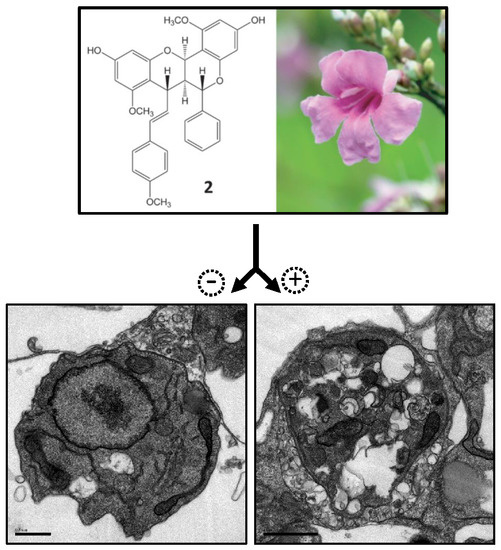
2.3. Electron Microscopy
2.4. In Vivo Infection
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation of Compounds Brachydin A (1), Brachydin B (2), and Brachydin C (3)
4.3. Mice
4.4. Parasites
4.5. Activity of Dimeric Flavonoids Against Axenic Promastigotes of Leishmania
4.6. Cytotoxicity Assay
4.7. In Vitro Macrophage Infection with L. amazonensis
4.8. Image Acquisition
4.9. Automated Image Analysis
4.10. Calculation of Biological Response
4.11. Drug Combination
4.12. Transmission Electron Microscopy
4.13. In Vivo Infection with L. amazonensis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; The WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- González, B.; Suárez-Roca, H.; Bravo, A.; Salas-Auvert, R.; Avila, D. Chemical composition and biological activity of extracts from arrabidaea bilabiata. Pharm. Biol. 2000, 38, 287–290. [Google Scholar] [CrossRef]
- Martin, F.; Hay, A.-E.; Cressend, D.; Reist, M.; Vivas, L.; Gupta, M.P.; Carrupt, P.-A.; Hostettmann, K. Antioxidant C-glucosylxanthones from the leaves of Arrabidaea patellifera. J. Nat. Prod. 2008, 71, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.P.V.; Oliveira, A.B.; Lombardi, J.A.; Filho, J.D.S.; Chiari, E. Trypanocidal activity of triterpenes from Arrabidaea triplinervia and derivatives. Biol. Pharm. Bull. 2006, 29, 2307–2309. [Google Scholar] [CrossRef]
- da Rocha, C.Q.; Vilela, F.C.; Cavalcante, G.P.; Santa-Cecília, F.V.; Santos-e-Silva, L.; dos Santos, M.H.; Giusti-Paiva, A. Anti-inflammatory and antinociceptive effects of Arrabidaea brachypoda (DC.) Bureau roots. J. Ethnopharmacol. 2011, 133, 396–401. [Google Scholar] [CrossRef]
- da Rocha, C.Q.; Queiroz, E.F.; Meira, C.S.; Moreira, D.R.M.; Soares, M.B.P.; Marcourt, L.; Vilegas, W.; Wolfender, J.-L. Dimeric flavonoids from Arrabidaea brachypoda and assessment of their anti-Trypanosoma cruzi activity. J. Nat. Prod. 2014, 77, 1345–1350. [Google Scholar] [CrossRef]
- Devakaram, R.; Black, D.S.; Andrews, K.T.; Fisher, G.M.; Davis, R.A.; Kumar, N. Synthesis and antimalarial evaluation of novel benzopyrano[4,3-b]benzopyran derivatives. Bioorg. Med. Chem. 2011, 19, 5199–5206. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira-Neto, J.L.; Moon, S.; Jang, J.; Yang, G.; Lee, C.; Moon, H.K.; Chatelain, E.; Genovesio, A.; Cechetto, J.; Freitas-Junior, L.H. An image-based high-content screening assay for compounds targeting intracellular Leishmania donovani amastigotes in human macrophages. PLoS Negl. Trop. Dis. 2012, 6, e1671. [Google Scholar] [CrossRef]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: Recent progress and challenges in assay development. Drug Discov. Today 2017, 22, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, D.; Smith, D.; Opperdoes, F.; Stern, S.; Olafson, R.W.; Zilberstein, D. Retooling Leishmania metabolism: From sand fly gut to human macrophage. FASEB J. 2008, 22, 590–602. [Google Scholar] [CrossRef]
- Saxena, A.; Lahav, T.; Holland, N.; Aggarwal, G.; Anupama, A.; Huang, Y.; Volpin, H.; Myler, P.J.; Zilberstein, D. Analysis of the Leishmania donovani transcriptome reveals an ordered progression of transient and permanent changes in gene expression during differentiation. Mol. Biochem. Parasitol. 2007, 152, 53–65. [Google Scholar] [CrossRef]
- Burchmore, R.J.; Barrett, M.P. Life in vacuoles--nutrient acquisition by Leishmania amastigotes. Int. J. Parasitol. 2001, 31, 1311–1320. [Google Scholar] [CrossRef]
- Vermeersch, M.; da Luz, R.I.; Toté, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef]
- De Muylder, G.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef]
- Jaramillo, M.; Gomez, M.A.; Larsson, O.; Shio, M.T.; Topisirovic, I.; Contreras, I.; Luxenburg, R.; Rosenfeld, A.; Colina, R.; McMaster, R.W.; et al. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell. Host. Microbe. 2011, 9, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Buates, S.; Matlashewski, G. Treatment of experimental leishmaniasis with the immunomodulators imiquimod and S-28463: Efficacy and mode of action. J. Infect. Dis. 1999, 179, 1485–1494. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, W.; Simeonov, A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol. 2018, 175, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Borges, V.M.; Vannier-Santos, M.A.; de Souza, W. Subverted transferrin trafficking in Leishmania-infected macrophages. Parasitol. Res. 1998, 84, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Reiner, N.E. Exosomes and other microvesicles in infection biology: Organelles with unanticipated phenotypes. Cell. Microbiol. 2011, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Courret, N.; Lang, T.; Milon, G.; Antoine, J.C. Intradermal inoculations of low doses of Leishmania major and Leishmania amazonensis metacyclic promastigotes induce different immunoparasitic processes and status of protection in BALB/c mice. Int. J. Parasitol. 2003, 33, 1373–1383. [Google Scholar] [CrossRef]
- Mears, E.R.; Modabber, F.; Don, R.; Johnson, G.E. A Review: The Current In Vivo Models for the Discovery and Utility of New Anti-leishmanial Drugs Targeting Cutaneous Leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003889. [Google Scholar] [CrossRef]
- Khare, S.; Nagle, A.S.; Biggart, A.; Lai, Y.H.; Liang, F.; Davis, L.C.; Barnes, S.W.; Mathison, C.J.N.; Myburgh, E.; Gao, M.-Y.; et al. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature 2016, 537, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.N.; Calabrich, A.F.d.C.; Tavares, R.d.S.; Wietzerbin, J.; de Freitas, L.A.R.; Veras, P.S.T. Differential properties of CBA/J mononuclear phagocytes recovered from an inflammatory site and probed with two different species of Leishmania. Microbes. Infect. 2003, 5, 251–260. [Google Scholar] [CrossRef]
- Tegazzini, D.; Díaz, R.; Aguilar, F.; Peña, I.; Presa, J.L.; Yardley, V.; Martin, J.J.; Coteron, J.M.; Croft, S.L.; Cantizani, J. A Replicative In vitro Assay for Drug Discovery against Leishmania donovani. Antimicrob. Agents Chemother. 2016, 60, 3524–3532. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |







| IC50 (µM) Promastigotes Leishmania sp. | IC50 Amastigotes | |||||
|---|---|---|---|---|---|---|
| Compound | L. amazonensis | L. braziliensis | L. infantum | CC50 MΦ | IC50 L. amazonensis | SI |
| (1) | >20 | >20 | >20 | >20 | >20 | NC |
| (2) | 9.16 ± 1 | 7.05 ± 1 | 12.90 ± 3 | >20 | 2.20 ± 0.09 | 9.1 |
| (3) | 10 ± 0.80 | 8.82 ± 3 | 18.36 ± 2 | >20 | 6.25 ± 1.28 | 3.2 |
| AMB | 0.14 ± 0.01 | 0.11 | 0.05 | >20 | 0.10 ± 0.02 | 200 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, V.P.C.; Quintino da Rocha, C.; Ferreira Queiroz, E.; Marcourt, L.; Vilegas, W.; Grimaldi, G.B.; Furrer, P.; Allémann, É.; Wolfender, J.-L.; Soares, M.B.P. Antileishmanial Activity of Dimeric Flavonoids Isolated from Arrabidaea brachypoda. Molecules 2019, 24, 1. https://doi.org/10.3390/molecules24010001
Rocha VPC, Quintino da Rocha C, Ferreira Queiroz E, Marcourt L, Vilegas W, Grimaldi GB, Furrer P, Allémann É, Wolfender J-L, Soares MBP. Antileishmanial Activity of Dimeric Flavonoids Isolated from Arrabidaea brachypoda. Molecules. 2019; 24(1):1. https://doi.org/10.3390/molecules24010001
Chicago/Turabian StyleRocha, Vinícius P. C., Cláudia Quintino da Rocha, Emerson Ferreira Queiroz, Laurence Marcourt, Wagner Vilegas, Gabriela B. Grimaldi, Pascal Furrer, Éric Allémann, Jean-Luc Wolfender, and Milena B. P. Soares. 2019. "Antileishmanial Activity of Dimeric Flavonoids Isolated from Arrabidaea brachypoda" Molecules 24, no. 1: 1. https://doi.org/10.3390/molecules24010001