Novel Methinic Functionalized and Dendritic C-Scorpionates
Abstract
:1. Introduction
2. Results
2.1. Functionalization at the Methinic Carbon
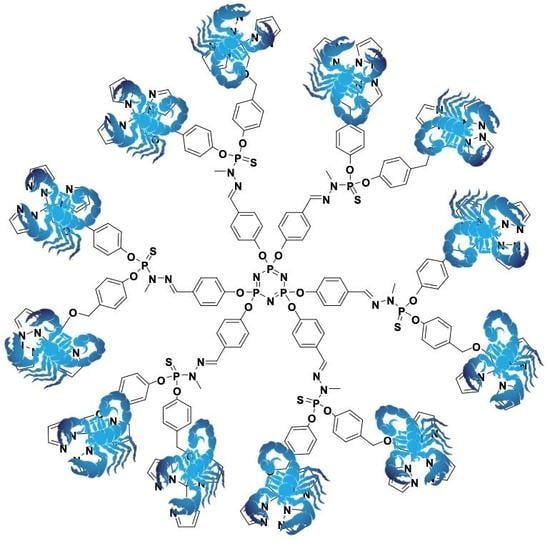
2.2. Dendritic Functionalization
- the nucleophilic substitution of the core N3P3Cl6 with 6 equivalents of previously prepared sodium salt of 4-hydroxybenzaldehyde (spacer), led to a sort of “extended core”. This product is not yet a dendrimer, since the branching points were not multiplied but stayed the same;
- the “branching molecule” was obtained from monomethylhydrazine with thiophosphonylchloride (solubilized in chloroform);
- six equivalents of N-methylhydrazido thiophosphonyldichloride are condensed with the aldehydic terminations of the macromolecule to yield the first-generation phosphorus dendrimer.
2.3. Catalytic Studies
3. Materials and Methods
3.1. Synthesis of Odium tris-2,2,2-(pyrazol-1-yl)ethanoate, NaOCH2C(pz)3 (2), (pz = pyrazolyl)
3.2. Synthesis of (tris-2,2,2-(pyrazol-1-yl)ethoxy)benzyl, PhCH2OCH2C(pz)3 (3), (pz = pyrazolyl)
3.3. Synthesis of 4-(2,2,2-tris(1-pyrazolyl)ethoxymethyl)phenol, 4-OH-C6H4CH2OCH2C(pz)3 (4) (pz = pyrazolyl)


3.4. Synthesis of N-tosyl-2-(tris-2,2,2-(1-pyrazolyl)ethoxy)ethaneamine, TsNHCH2CH2OCH2C(pz)3 (5) (Ts = para-toluenesulfonyl, pz = pyrazolyl)
3.5. Synthesis of TsNHCH2CH2TsNCH2CH2OCH2C(pz)3 (6) (Ts = para-toluenesulfonyl, pz = pyrazolyl)
3.6. Synthesis of the Scorpionate Dendrimer, N3P3-Gc1-[CH2C(pz)3]12 (7)

3.7. Catalytic tests
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guilard, R.; Erker, G.; Raithby, P.; Xu, Q. The diversity of coordination chemistry: A special issue in honor of Prof. Pierre Braunstein. Coord. Chem. Rev. 2017, 350, 1–2. [Google Scholar] [CrossRef]
- Pettinari, C. Scorpionates II: Chelating Borate Ligands-Dedicated to Swiatoslaw Trofimenko; Imperial College Press: London, UK, 2008; ISBN-13-1-86094-876-3. [Google Scholar]
- Trofimenko, S. Scorpionates: The Coordination Chemistry of Polypyrazolylborates Ligands; Imperial College Press: London, UK, 1999; ISBN-1-86094-172-9. [Google Scholar]
- Pettinari, C.; Santini, C. Comprehensive Coordination Chemistry II: From Biology to Nanotechnology; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier-Pergamon (APS): Amsterdam, The Netherlands, 2003; Volume 1, pp. 159–172. [Google Scholar]
- Pettinari, C.; Pettinari, R. Metal derivatives of poly(pyrazolyl)alkanesi. Tris(pyrazolyl)alkanes and related systems. Coord. Chem. Rev. 2005, 249, 525–543. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 2236–2252. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S. C-homoscorpionate oxidation catalysts—Electrochemical and catalytic activity. Catalysts 2017, 7, 12. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Carbon dioxide-to-methanol single-pot conversion using a C-scorpionate iron(II) catalyst. Green Chem. 2017, 19, 4801–4962. [Google Scholar] [CrossRef]
- Mendes, M.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Liquid phase oxidation of xylenes catalyzed by the tripodal C-scorpionate iron(II) complex [FeCl2{κ3-HC(pz)3}]. Polyhedron 2017, 125, 151–155. [Google Scholar] [CrossRef]
- Bigmore, H.R.; Lawrence, S.C.; Mountford, P.; Tredget, C.S. Coordination, organometallic and related chemistry of tris(pyrazolyl)methane ligands. Dalton Trans. 2005, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Wanke, R.; Smolenski, P.; da Silva, M.F.G.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Cu(I) complexes bearing the new sterically demanding and coordination flexible tris(3-phenyl-1-pyrazolyl)methanesulfonate ligand and the water-soluble phosphine 1,3,5-triaza-7-phosphaadamantane or related ligands. Inorg. Chem. 2008, 47, 10158–10168. [Google Scholar] [CrossRef] [PubMed]
- Mehn, M.P.; Fujisawa, K.; Hegg, E.L.; Que, L. Oxygen activation by nonheme iron(II) complexes: α-Keto carboxylate versus carboxylate. J. Am. Chem. Soc. 2003, 125, 7828–7842. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Semeniuc, R.F.; Little, C.A.; Smith, M.D. Synthesis of open and closed metallacages using novel tripodal ligands: unusually stable silver(i) inclusion compound. Inorg. Chem. 2006, 45, 7758–7769. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Efficient cyclohexane oxidation with hydrogen peroxide catalysed by a C-scorpionate iron(II) complex immobilized on desilicated MOR zeolite. Appl. Catal. A: Gen. 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; de Almeida, M.P.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-scorpionate Fe(II) complex in carbon materials for cyclohexane oxidation with hydrogen peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- De Almeida, M.P.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Highly efficient and reusable CNT supported iron(II) catalyst for microwave assisted alcohol oxidation. Dalton Trans. 2016, 45, 6816–6819. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Tuning cyclohexane oxidation: Combination of microwave irradiation and ionic liquid with the C-scorpionate [FeCl2(Tpm)] catalyst. Organometallics 2017, 36, 192–198. [Google Scholar] [CrossRef]
- Wang, J.; Martins, L.M.D.R.S.; Ribeiro, A.P.C.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Supported C-scorpionate vanadium(IV) complexes as reusable catalysts for xylene oxidation. Chem. Asian J. 2017, 12, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Buijnsters, J.G.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenised C-scorpionate iron(II) complex on nanostructured carbon materials as catalysts for microwave-assisted oxidation reactions. ChemCatChem 2018, 10, 1821–1828. [Google Scholar] [CrossRef]
- Van-Dúnem, V.; Carvalho, A.P.; Martins, L.M.D.R.S.; Martins, A. Improved cyclohexane oxidation catalyzed by a heterogenised iron(II) complex on hierarchical Y zeolite through surfactant mediated technology. ChemCatChem 2018, 10, 4058–4066. [Google Scholar] [CrossRef]
- Benisvy, L.; Wanke, R.; Kuznetsov, M.L.; da Silva, M.; Pombeiro, A.J.L. Towards the functionalization of the methine carbon of a sterically hindered tris(pyrazolyl)methane: Is a radical pathway envisageable? Synthesis and structure of tetrakis(3,5-dimethylpyrazolyl)methane. Tetrahedron 2009, 65, 9218–9223. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Figueiredo, J.L. Commercial gold(I) and gold(III) compounds supported on carbon materials as greener catalysts for the oxidation of alkanes and alcohols. ChemCatChem 2018, 10, 1804–1813. [Google Scholar] [CrossRef]
- Mishra, G.S.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Highly active and selective supported rhenium catalysts for aerobic oxidation of n-hexane and n-heptane. Catalysts 2018, 8, 114. [Google Scholar] [CrossRef]
- Mishra, G.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Cyclohexane oxidation with dioxygen catalyzed by supported pyrazole rhenium complexes. J. Mol. Cat. A: Chem. 2008, 285, 92–100. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Silva, M.F.C.G.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Silva, T.F.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Org. Chem. 2011, 24, 764–773. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Turrin, C.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. (Eds.) Dendrimers: Towards Catalytic, Material and Biomedical Uses; John Wiley & Sons Ltd.: Chichester, UK, 2011. [Google Scholar]
- Caminade, A.-M.; Servin, P.; Laurent, R.; Majoral, J.P. Dendrimeric phosphines in asymmetric catalysis. Chem. Soc. Rev. 2008, 37, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.L.; Grattan, T.C.; Brown, K.J.; Little, C.A.; Lamba, J.J.S.; Rheingold, A.L.; Sommer, R.D. Syntheses of tris(pyrazolyl)methane ligands and {[tris(pyrazolyl)methane]Mn(CO)3} SO3CF3 complexes: Comparison of ligand donor properties. J. Organomet. Chem. 2000, 607, 120–128. [Google Scholar] [CrossRef]
- Doucet, H.; Hierso, J.-C. Palladium-based catalytic systems for the synthesis of conjugated enynes by sonogashira reactions and related alkynylations. Angew. Chem. Int. Ed. 2007, 46, 834–871. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S.; Phillips, A.M.F.; Pombeiro, A.J.L. C-C bond formation in sustainable synthesis of pharmaceuticals. In Sustainable Synthesis of Pharmaceuticals: Using Transition Metal Complexes as Catalysts; Pereira, M.M., Calvete, M.J.F., Eds.; Green Chemistry Series; RSC Publishers: Cambridge, UK, 2018; pp. 193–229, ISBN-10:1782629343; ISBN-13:978-1782629344. [Google Scholar]
- Chinchilla, R.; Najera, C. The sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Prashad, M. Palladium-catalyzed heck arylations in the synthesis of active pharmaceutical ingredients. Top. Organomet. Chem. 2004, 6, 181–203. [Google Scholar] [CrossRef]
- Reger, D.L.; Wright, T.D.; Semeniuc, R.F.; Grattan, T.C.; Smith, M.D. Supramolecular structures of cadmium(II) coordination polymers: A new class of ligands formed by linking tripodal tris(pyrazolyl)methane units. Inorg. Chem. 2001, 40, 6212–6219. [Google Scholar] [CrossRef] [PubMed]
- Moss, T.A.; Barber, D.M.; Kyle, A.F.; Dixon, D.J. Catalytic asymmetric alkylation reactions for the construction of protected ethylene-amino and propylene-amino motifs attached to quaternary stereocentres. Chem. Eur. J. 2013, 19, 3071–30814. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.E.; Ford, T.M.; Bulkowski, J.E. Synthesis of selectively protected tri- and hexaamine macrocycles. J. Org. Chem. 1982, 47, 412–415. [Google Scholar] [CrossRef]
- Greene, T.W.; Wuts, P.G.M. Chemistry-Protective Groups in Organic Synthesis, 3rd ed.; Wiley-Interscience Publication: New York, NY, USA, 1999. [Google Scholar]
- Li, Z.; Zhou, Z.; Wang, L.; Zhou, Q.; Tang, C. Enantioselective reaction of secondary alcohols with phthalimide in the presence of a chiral tri-coordinate phosphorus reagent in Mitsunobu reaction. Tetrahedron: Asymmetry 2002, 13, 145–148. [Google Scholar] [CrossRef]
- Sen, S.E.; Roach, S.L. A convenient two-step procedure for the synthesis of substituted allylic amines from allylic alcohols. Synthesis 1995, 756–758. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Cerquetella, A.; Cingolani, A.; Pettinari, R.; Monari, M.; Wanke, R.; Kuznetsov, M.L.; Pombeiro, A.J.L. Switching between κ2 and κ3 Bis(pyrazol-1-yl)acetate ligands by tuning reaction conditions: Synthesis, spectral, electrochemical, structural, and theoretical studies on arene-Ru(II) derivatives of bis(azol-1-yl)acetate Ligands. Inorg. Chem. 2009, 48, 6096–6108. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.; Martins, L.M.D.R.S.; Mahmudov, K.T.; Kopylovich, M.N.; Drew, M.G.B.; Pettinari, C.; Pombeiro, A.J.L. Microwave-assisted and solvent-free peroxidative oxidation of 1-phenylethanol to acetophenone with a Cu(II)-TEMPO catalytic system. Catal. Commun. 2014, 48, 4048–4058. [Google Scholar] [CrossRef]
- Majoral, J.P.; Caminade, A.M. What to do with phosphorus in dendrimer chemistry. In New Aspects in Phosphorus Chemistry II; Springer: Berlin, Germany, 2003; Volume 223, p. 111. [Google Scholar]
- Slany, M.; Bardaji, M.; Casanove, M.J.; Caminade, A.M.; Majoral, J.P.; Chaudret, B. Dendrimer surface chemistry. Facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.M.; Majoral, J.P. Synthesis of bowl-shaped dendrimers from generation 1 to generation 8. J. Organomet. Chem. 1997, 529, 51–58. [Google Scholar] [CrossRef]
- Rull, J.; Jara, J.J.; Sebastian, R.M.; Vallribera, A.; Najera, C.; Majoral, J.P.; Caminade, A.M. Recoverable dendritic phase-transfer catalysts containing (+)-cinchonine-derived ammonium salts. ChemCatChem 2016, 8, 2049–2056. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-H.; Zhang, X.-D.; Xie, Y.-X. Efficient and copper-free sonogashira cross-coupling reaction catalyzed by Pd(OAc)2/Pyrimidines catalytic system. Eur. J. Org. Chem. 2005, 4256–4259. [Google Scholar] [CrossRef]
- Touj, N.; Yaşar, S.; Ozdemir, N.; Hamdi, N.; Ozdemir, I. Sonogashira cross-coupling reaction catalysed by mixed NHC-Pd-PPh3 complexes under copper free conditions. J. Organomet. Chem. 2018, 860, 59–71. [Google Scholar] [CrossRef]
- Phanopoulos, A.; White, A.J.P.; Long, N.J.; Miller, P.W. Catalytic transformation of levulinic acid to 2-methyltetrahydrofuran using ruthenium–n-triphos complexes. ACS Catal. 2015, 5, 2500–2512. [Google Scholar] [CrossRef]
- Keller, M.; Hameau, A.; Spataro, G.; Ladeira, S.; Caminade, A.M.; Majoral, J.P.; Ouali, A. An efficient and recyclable dendritic catalyst able to dramatically reduce palladium leaching in Suzuki couplings. Green Chem. 2012, 14, 2807–2815. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Cerquetella, A.; Pettinari, R.; Monari, M.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Coordination chemistry of the (h6-cymene)ruthenium(II) fragment with bis-, tris-, and tetrakis(pyrazol-1-yl)borate ligands: Synthesis, structural, electrochemical and catalytic diastereoselective nitroaldol reaction studies. Organometallics 2011, 30, 1616–1626. [Google Scholar] [CrossRef]
- Rocha, B.G.M.; Mac Leod, T.C.O.; Guedes da Silva, M.F.C.; Luzyanin, K.V.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. NiII, CuII and ZnII complexes with a sterically hindered scorpionate ligand (TpmsPh) and catalytic application in the diasteroselective nitroaldol (henry) reaction. Dalton Trans. 2014, 43, 15192–15200. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds are not available from the authors. |












© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, L.M.D.R.S.; Wanke, R.; Silva, T.F.S.; Pombeiro, A.J.L.; Servin, P.; Laurent, R.; Caminade, A.-M. Novel Methinic Functionalized and Dendritic C-Scorpionates. Molecules 2018, 23, 3066. https://doi.org/10.3390/molecules23123066
Martins LMDRS, Wanke R, Silva TFS, Pombeiro AJL, Servin P, Laurent R, Caminade A-M. Novel Methinic Functionalized and Dendritic C-Scorpionates. Molecules. 2018; 23(12):3066. https://doi.org/10.3390/molecules23123066
Chicago/Turabian StyleMartins, Luísa M. D. R. S., Riccardo Wanke, Telma F. S. Silva, Armando J. L. Pombeiro, Paul Servin, Régis Laurent, and Anne-Marie Caminade. 2018. "Novel Methinic Functionalized and Dendritic C-Scorpionates" Molecules 23, no. 12: 3066. https://doi.org/10.3390/molecules23123066