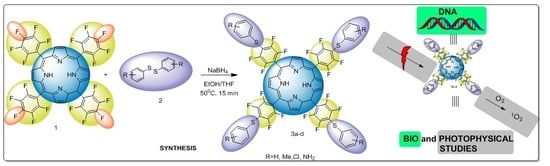
A New Protocol for the Synthesis of New Thioaryl-Porphyrins Derived from 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: Photophysical Evaluation and DNA-Binding Interactive Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Absorption and Emission Properties of Thioaryl-Porphyrins 3a–d
2.2. Singlet Oxygen Generation (1O2) Experiments
2.3. Biomolecule Interactive Studies
2.3.1. DNA-Binding Assays by Absorption and Emission Analysis
2.3.2. DNA Molecular Docking with Thioaryl-Porphyrins 3a–d
3. Materials and Methods
General Procedure for the Synthesis 3a–d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slomp, A.M.; Barreira, S.M.W.; Carrenho, L.Z.B.; Vandresen, C.C.; Zattoni, I.F.; Ló, S.M.S.; Dallagnol, J.C.C.; Ducatti, D.R.B.; Orsato, A.; Duarte, M.E.R.; et al. Photodynamic effect of meso-(aryl)porphyrins and meso-(1-methyl-4-pyridinium)porphyrins on HaCaT keratinocytes. Bioorg. Med. Chem. Lett. 2017, 27, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Fazekas, M.; Notaras, E.G.A.; Blau, W.J.; Zawadzka, M.; Locos, O.B.; Ni Mhuircheartaigh, E.M. Nonlinear optical properties of porphyrins. Adv. Mater. 2007, 19, 2737–2774. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerene-porphyrin architectures; photosynthetic antenna and reaction center models. Chem. Soc. Rev. 2002, 31, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Figueira, F.; Mendes, R.F.; Cavaleiro, J.A.S.; da Neves, M.G.P.M.S.; Simões, M.M.Q.; Almeida Paz, F.A.; Tomé, J.P.C.; Nakagaki, S. Copper–porphyrin–metal–organic frameworks as oxidative heterogeneous catalysts. ChemCatChem 2017, 9, 2939–2945. [Google Scholar] [CrossRef]
- Stich, M.I.J.; Fischer, L.H.; Wolfbeis, O.S. Multiple fluorescent chemical sensing and imaging. Chem. Soc. Rev. 2010, 39, 3102–3114. [Google Scholar] [CrossRef] [PubMed]
- Drain, C.M.; Russell, K.C.; Lehn, J.M. Self-assembly of a multi-porphyrin supramolecular macrocycle by hydrogen bond molecular recognition. Chem. Commun. 1996, 337–338. [Google Scholar] [CrossRef]
- Walter, M.G.; Rudine, A.B.; Wamser, C.C. Porphyrins and phthalocyanines in solar photovoltaic cells. J. Porphyr. Phthalocyanines 2010, 14, 759–792. [Google Scholar] [CrossRef]
- Quiroz-Segoviano, R.I.Y.; Serratos, I.N.; Rojas-González, F.; Tello-Solís, S.R.; Sosa-Fonseca, R.; Medina-Juárez, O.; Menchaca-Campos, C.; García-Sánchez, M.A. On tuning the fluorescence emission of porphyrin free bases bonded to the pore walls of organo-modified silica. Molecules 2014, 19, 2261–2285. [Google Scholar] [CrossRef] [PubMed]
- George, C.D.; Richardson, T.; Hofton, M.E.; Vale, C.M. Chlorine gas sensing using thin films of meso-tetra ž p-stearamidophenyl/porphyrin. Mater. Sci. Eng. 1999, 8–9, 559–563. [Google Scholar] [CrossRef]
- Costa, J.I.T.; Tomé, A.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: A versatile platform to novel porphyrinic materials. J. Porphyr. Phthalocyanines 2011, 15, 1116–1133. [Google Scholar] [CrossRef]
- Aratani, N.; Takagi, A.; Yanagawa, Y.; Matsumoto, T.; Kawai, T.; Yoon, Z.S.; Kim, D.; Osuka, A. Giant meso-meso-linked porphyrin arrays of micrometer molecular length and their fabrication. Chem. A Eur. J. 2005, 11, 3389–3404. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, N.V.S.D.K.; Hu, X.; Zhou, Z.; Fronczek, F.R.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Vicente, M.G.H. Synthesis and in vitro evaluation of BBB permeability, tumor cell uptake, and cytotoxicity of a series of carboranylporphyrin conjugates. J. Med. Chem. 2014, 57, 6718–6728. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.R.; Silva, S.; Bispo, M.; Zuzarte, M.; Gomes, C.; Girão, H.; Cavaleiro, J.A.S.; Ribeiro, C.A.F.; Tomé, J.P.C.; Fernandes, R. Mitochondria-targeted photodynamic therapy with a galactodendritic chlorin to enhance cell death in resistant bladder cancer cells. Bioconjug. Chem. 2016, 27, 2762–2769. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S. Cancer, photodynamic therapy and porphyrin-type derivatives. An. Acad. Bras. Cienc. 2018, 90, 993–1026. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, P.M.R.; Carvalho, J.J.; Silva, S.; Cavaleiro, J.A.S.; Schneider, R.J.; Fernandes, R.; Tomé, J.P.C. Porphyrin conjugated with serum albumins and monoclonal antibodies boosts efficiency in targeted destruction of human bladder cancer cells. Org. Biomol. Chem. 2014, 12, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.R.; Silva, S.; Ramalho, J.S.; Gomes, C.M.; Girão, H.; Cavaleiro, J.A.S.; Ribeiro, C.A.F.; Tomé, J.P.C.; Fernandes, R. The role of galectin-1 in in vitro and in vivo photodynamic therapy with a galactodendritic porphyrin. Eur. J. Cancer 2016, 68, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Dias, S.R.S.; Carvalho, C.M.B.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; et al. Mechanisms of photodynamic inactivation of a Gram-negative recombinant bioluminescent bacterium by cationic porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial photodynamic therapy against endodontic Enterococcus faecalis and Candida albicans mono and mixed biofilms in the presence of photosensitizers: A comparative study with classical endodontic irrigants. Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marciel, L.; Teles, L.; Moreira, B.; Pacheco, M.; Lourenço, L.M.O.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Faustino, M.A.F.; Almeida, A. An effective and potentially safe blood disinfection protocol using tetrapyrrolic photosensitizers. Future Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting current photoactive materials for antimicrobial photodynamic therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Day, R.A.; Estabrook, D.A.; Logan, J.K.; Sletten, E.M. Fluorous photosensitizers enhance photodynamic therapy with perfluorocarbon nanoemulsions. Chem. Commun. 2017, 53, 13043–13046. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.D.; Silva, J.A.; Fonseca, S.M.; Arranja, C.T.; Urbano, A.M.; Sobral, A.J.F.N. Photophysical characterization and in vitro phototoxicity evaluation of 5,10,15,20-tetra(quinolin-2-yl)porphyrin as a potential sensitizer for photodynamic therapy. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Chitgupi, U.; Lovell, J.F.; Rajendiran, V. Assessing photosensitizer targeting using meso-tetra(Carboxyphenyl)porphyrin. Molecules 2018, 23, 892. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jiang, Y.Y.; Jiang, T.; Yin, W.; Yang, J.P.; Cao, M.L.; Fang, Y.Q.; Liu, H.Y. Water-soluble manganese and iron mesotetrakis(carboxyl)porphyrin: DNA binding, oxidative cleavage, and cytotoxic activities. Molecules 2017, 22, 1084. [Google Scholar] [CrossRef] [PubMed]
- Garfias-Gonzalez, K.I.; Organista-Mateos, U.; Borja-Miranda, A.; Gomez-Vidales, V.; Hernandez-Ortega, S.; Cortez-Maya, S.; Martínez-García, M. High fluorescent porphyrin-PAMAM-fluorene dendrimers. Molecules 2015, 20, 8548–8559. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.N.; Galmiche, A.; Tomé, J.P.C.; Boullier, A.; Neves, M.G.P.M.S.; Silva, E.M.P.; Capiod, J.C.; Cavaleiro, J.A.S.; Santus, R.; Mazière, J.C.; et al. Chain-dependent photocytotoxicity of tricationic porphyrin conjugates and related mechanisms of cell death in proliferating human skin keratinocytes. Biochem. Pharmacol. 2010, 80, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Castro, K.A.D.F.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Ribeiro, R.R.; Wypych, F.; Nakagaki, S. Synthesis of new metalloporphyrin derivatives from [5,10,15,20-tetrakis (pentafluorophenyl)porphyrin] and 4-mercaptobenzoic acid for homogeneous and heterogeneous catalysis. Appl. Catal. A Gen. 2015, 503, 9–19. [Google Scholar] [CrossRef]
- Breslow, R.; Gabriele, B.; Yang, J. Geometrically directed selective steroid hydroxylation with high turnover by a fluorinated artificial cytochrome P-450. Tetrahedron Lett. 1998, 39, 2887–2890. [Google Scholar] [CrossRef]
- Battioni, P.; Brigaud, O.; Desvaux, H.; Mansuy, D.; Traylor, T.G. Preparation of functionalized polyhalogenated tetraaryl-porphyrins by selective substitution of the p-Fluorines of meso-tetra-(pentafluorophenyl)porphyrins. Tetrahedron Lett. 1991, 32, 2893–2896. [Google Scholar] [CrossRef]
- Auras, B.L.; Meller, S.L.; da Silva, M.P.; Neves, A.; Cocca, L.H.Z.; De Boni, L.; da Silveira, C.H.; Iglesias, B.A. Synthesis, spectroscopic/electrochemical characterization and DNA interaction study of novel ferrocenyl-substituted porphyrins. Appl. Organomet. Chem. 2018, 32, 1–12. [Google Scholar] [CrossRef]
- Azenha, E.G.; Serra, A.C.; Pineiro, M.; Pereira, M.M.; Melo, J.S.; Arnaut, L.G.; Formosinho, S.J.; Gonsalves, A.M.R. Heavy-atom effects on metalloporphyrins and polyhalogenated porphyrins. Chem. Phys. 2002, 280, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.J.P.; Pina, J.; Pereira, M.M.; Arnaut, L.G. On the singlet states of porphyrins, chlorins and bacteriochlorins and their ability to harvest red/infrared light. Photochem. Photobiol. Sci. 2012, 11, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.C.; Silva, S.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A.; Cavaleiro, J.A.S.; Tomé, J.P.C.; Cunha, Â. Cationic galactoporphyrin photosensitisers against UV-B resistant bacteria: Oxidation of lipids and proteins by 1O2. Photochem. Photobiol. Sci. 2013, 12, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, R.C.; Auras, B.L.; de Souza, B.; Neves, A.; Nunes, F.S.; Cocca, L.H.Z.; de Boni, L.; Iglesias, B.A. Synthesis, photophysical properties and spectroelectrochemical characterization of 10-(4-methyl-bipyridyl)-5,15-(pentafluorophenyl)corrole. J. Photochem. Photobiol. A Chem. 2017, 332, 306–315. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wohrle, D.; Hackbarth, S.; Roder, B.; Schnurpfeil, G. Singlet oxyg en quantum yields of different photo- sensitizers in polar solvents and mic ellar solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Preuß, A.; Saltsman, I.; Mahammed, A.; Pfitzner, M.; Goldberg, I.; Gross, Z.; Röder, B. Photodynamic inactivation of mold fungi spores by newly developed charged corroles. J. Photochem. Photobiol. B Biol. 2014, 133, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Grancho, J.C.P.; Pereira, M.M.; da Miguel, M.G.; Rocha, G.A.M.; Burrows, H.D. Synthesis, spectra and photophysics of some free base tetrafluoroalkyl and tetrafluoroaryl porphyrins with potential applications in imaging. Photochem. Photobiol. 2002, 75, 249–256. [Google Scholar] [CrossRef]
- Pineiro, M.; Pereira, M.M.; Rocha Gonsalves, A.M.D.A.; Arnaut, L.G.; Formosinho, S.J. Singlet oxygen quantum yields from halogenated chlorins: Potential new photodynamic therapy agents. J. Photochem. Photobiol. A Chem. 2001, 138, 147–157. [Google Scholar] [CrossRef]
- Cavaleiro, J.A.S.; Görner, H.; Lacerda, P.S.S.; MacDonald, J.G.; Mark, G.; Neves, M.G.P.M.S.; Nohr, R.S.; Schuchmann, H.P.; Von Sonntag, C.; Tomé, A.C. Singlet oxygen formation and photostability of meso-tetraarylporphyrin derivatives and their copper complexes. J. Photochem. Photobiol. A Chem. 2001, 144, 131–140. [Google Scholar] [CrossRef]
- Pineiro, M.; Carvalho, A.L.; Pereira, M.M.; Rocha Gonsalves, A.M.D.A.; Arnaut, L.G.; Formosinho, S.J. Photoacoustic measurements of porphyrin triplet-state quantum yields and singlet-oxygen efficiencies. Chem. A Eur. J. 1998, 4, 2299–2307. [Google Scholar] [CrossRef]
- Auras, B.L.; Oliveira, V.A.; Terenzi, H.; Neves, A.; Iglesias, B.A. Meso-Mono-[4-(1,4,7-triazacyclononanyl)]-tri(phenyl)]porphyrin and the respective zinc(ii)-complex: Complete characterization and biomolecules binding abilities. Photochem. Photobiol. Sci. 2016, 15, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Arba, M.; Tjahjono, D.H. The binding modes of cationic porphyrin-anthraquinone hybrids to DNA duplexes: In silico study. J. Biomol. Struct. Dyn. 2015, 33, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Krah, A.; Wien, F.; Garman, E.; McKenna, R.; Sanderson, M.; Neidle, S. A DNA-porphyrin minor-groove complex at atomic resolution: The structural consequences of porphyrin ruffling. Proc. Natl. Acad. Sci. USA 2000, 97, 9476–9481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef] [PubMed]
- Mocilac, P.; Osman, I.A.; Gallagher, J.F. Short C-H⋯F interactions involving the 2,5-difluorobenzene group: Understanding the role of fluorine in aggregation and complex C-F/C-H disorder in a 2 × 6 isomer grid. CrystEngComm. 2016, 18, 5764–5776. [Google Scholar] [CrossRef]
- Thalladi, V.R.; Weiss, H.C.; Bläser, D.; Boese, R.; Nangia, A.; Desiraju, G.R. C-H···F interactions in the crystal structures of some fluorobenzenes. J. Am. Chem. Soc. 1998, 120, 8702–8710. [Google Scholar] [CrossRef]
- Boer, D.R.; Canals, A.; Coll, M. DNA-binding drugs caught in action: The latest 3D pictures of drug-DNA complexes. Dalton Trans. 2009, 399–414. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |







 | ||
| Entry | Product | Yield (%) a |
| 1 |  | 84 |
| 2 |  | 92 |
| 3 |  | 82 |
| 4 |  | 93 |
| Porphyrin | nm, λ (log ε; M−1cm−1) a | Emission (nm) b | Φf c |
|---|---|---|---|
| 3a | 417 (5.00), 509 (3.81), 542 (3.00), 585 (3.30), 648 (2.60) | 648, 710 | 0.03 |
| 3b | 417 (5.04), 509 (3.85), 540 (3.04), 585 (3.34), 647 (2.65) | 648, 710 | 0.01 |
| 3c | 418 (5.02), 508 (3.86), 542 (3.08), 585 (3.36), 652 (2.62) | 652, 710 | 0.02 |
| 3d | 418 (5.07), 509 (3.96), 543 (3.30), 584 (3.52), 646 (2.69) | 649, 710 | 0.01 |
| Porphyrin | ΦΔ |
|---|---|
| 3a | 0.67 |
| 3b | 0.10 |
| 3c | 0.11 |
| 3d | 0.14 |
| TPP * | 0.66 |
| 1 | 0.70 a [39]; 0.80 b [40], 0.60 c [41] |
| Porphyrin | Absorption | Emission | ||||
|---|---|---|---|---|---|---|
| H(%) a | Δλ (nm) b | Kb (M−1) c | Q(%) d | KSV (M−1) e | kq (s−1M−1) f | |
| 3a | 7.65 | 0.0 | 2.08 × 106 | 32.58 | 4.92 × 103 | 2.14 × 1012 |
| 3b | 4.72 | 0.0 | 1.27 × 106 | 3.60 | 3.30 × 102 | 1.43 × 1011 |
| 3c | 3.22 | 0.0 | 0.76 × 106 | 21.28 | 2.66 × 102 | 1.15 × 1012 |
| 3d | 6.31 | 0.0 | 2.86 × 106 | 39.56 | 6.57 × 103 | 2.85 × 1012 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foletto, P.; Correa, F.; Dornelles, L.; A. Iglesias, B.; H. da Silveira, C.; A. Nogara, P.; T. da Rocha, J.B.; F. Faustino, M.A.; D. Rodrigues, O.E. A New Protocol for the Synthesis of New Thioaryl-Porphyrins Derived from 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: Photophysical Evaluation and DNA-Binding Interactive Studies. Molecules 2018, 23, 2588. https://doi.org/10.3390/molecules23102588
Foletto P, Correa F, Dornelles L, A. Iglesias B, H. da Silveira C, A. Nogara P, T. da Rocha JB, F. Faustino MA, D. Rodrigues OE. A New Protocol for the Synthesis of New Thioaryl-Porphyrins Derived from 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: Photophysical Evaluation and DNA-Binding Interactive Studies. Molecules. 2018; 23(10):2588. https://doi.org/10.3390/molecules23102588
Chicago/Turabian StyleFoletto, Patrícia, Fabiula Correa, Luciano Dornelles, Bernardo A. Iglesias, Carolina H. da Silveira, Pablo A. Nogara, João B. T. da Rocha, Maria A. F. Faustino, and Oscar E. D. Rodrigues. 2018. "A New Protocol for the Synthesis of New Thioaryl-Porphyrins Derived from 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: Photophysical Evaluation and DNA-Binding Interactive Studies" Molecules 23, no. 10: 2588. https://doi.org/10.3390/molecules23102588