Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Mussel-Inspired Hydrophilization
2.2. Characterization of Apatite-Coated PPBES
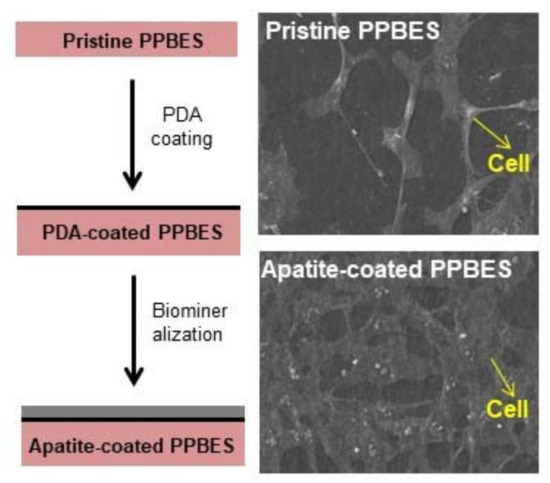
2.3. Cell Viability and Adhesion
3. Materials and Methods
3.1. Materials
3.2. Dopamine Polymerization and Biomineralization
3.3. Structural and Morphological Characterizations
3.4. In Vitro Cell Culture
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liao, K. Performance characterization and modeling of a composite hip-prosthesis. Exp. Tech. 1994, 18, 33–38. [Google Scholar] [CrossRef]
- Yu, S.C.; Hariram, K.P.; Kumar, R.; Cheang, P.; Aik, K.K. In vitro apatite formation and its growth kinetics on hydroxyapatite/polyetheretherketone biocomposites. Biomaterials 2005, 26, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Tsui, C.P.; Tang, C.Y.; Chow, C.L. Influence of interphase layer on the overall elasto-plastic behaviors of HA/PEEK biocomposite. Biomaterials 2004, 25, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Kirch, M.; Birchler, F.; Eckert, K.L.; Mayer, J.; Wintermantel, E.; Sittig, C.; PfundKlingenfuss, I.; Textor, M.; Spencer, N.D.; et al. Surface activation of polyetheretherketone (PEEK) and formation of calcium phosphate coatings by precipitation. J. Mater. Sci. Mater. Med. 1997, 8, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Wang, D.H.; Qiu, J.J.; Peng, F.; Liu, X.Y. Regulating the local pH level of titanium via Mg-Fe layered double hydroxides films for enhanced osteogenesis. Biomater. Sci. 2018, 6, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Black, C.; Gibbs, D.M.R.; Oreffo, R.O.C.; Brady, M.; Moshrefi-Torbati, M.; Yang, S.F. Characterization of new PEEK/HA composites with 3D HA network fabricated by extrusion freeforming. Molecules 2016, 21, 687. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Mayer, J.; Koch, B.; Wintermantel, E. Plasma-sprayed hydroxylapatite coating on carbon-fiber-reinforced thermoplastic composite-materials. J. Mater. Sci. Mater. Med. 1994, 5, 481–484. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.L.; Progar, D.J.; Johnson, W.S.; St Clair, T.L. Preliminary evaluation of hybrid titanium composite laminates. J. Adhes. 1995, 54, 223–240. [Google Scholar] [CrossRef]
- Li, D.; Lv, P.F.; Fan, L.F.; Huang, Y.Y.; Yang, F.; Mei, X.F.; Wu, D.C. The immobilization of antibiotic-loaded polymeric coatings on osteoarticular Ti implants for the prevention of bone infections. Biomater. Sci. 2017, 5, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.K.; Converse, G.L.; Kane, R.J.; Yue, W.M. Hydroxyapatite-reinforced polymer biocomposites for synthetic bone substitutes. JOM 2008, 60, 38–45. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium-phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, H.L.; Lee, K.M.; Baek, H.R.; Jin, K.; Hong, K.S.; Noh, J.H.; Lee, H.K. In vitro and in vivo evaluation of the bioactivity of hydroxyapatite-coated polyetheretherketone biocomposites created by cold spray technology. Acta Biomater. 2013, 9, 6177–6187. [Google Scholar] [CrossRef] [PubMed]
- Barkarmo, S.; Wennerberg, A.; Hoffman, M.; Kjellin, P.; Breding, K.; Handa, P.; Stenport, V. Nano-hydroxyapatite-coated peek implants: A pilot study in rabbit bone. J. Biomed. Mater. Res. Part A 2013, 101, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, A.; Sandukas, S. Processing and evaluation of bioactive coatings on polymeric implants. J. Biomed. Mater. Res. Part A 2013, 101, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, T.T. Current strategies to improve the bioactivity of peek. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Hahn, B.D.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Choi, J.H.; Kim, J.W.; Ahn, C.W.; Kim, H.E.; Yoon, B.H.; et al. Osteoconductive hydroxyapatite coated peek for spinal fusion surgery. Appl. Surf. Sci. 2013, 283, 6–11. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Xiong, C.D.; Zhang, S.L.; Li, X.Y.; Zhang, L.F. Bone-like apatite coating on functionalized poly(etheretherketone) surface via tailored silanization layers technique. Mater. Sci. Eng. C 2015, 55, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wang, F.; Han, Y. The structure, bond strength and apatite-inducing ability of micro-arc oxidized tantalum and their response to annealing. Appl. Surf. Sci. 2016, 361, 190–198. [Google Scholar] [CrossRef]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.B. Bio-inspired synthesis of minerals for energy, environment, and medicinal applications. Adv. Funct. Mater. 2013, 23, 10–25. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.X.; Lv, H.L.; Qin, Y.; Deng, L.H. Surface modification of chitosan film via polydopamine coating to promote biomineralization in bone tissue engineering. J. Bioact. Compat. Polym. 2018, 33, 134–145. [Google Scholar] [CrossRef]
- Zain, N.M.; Hussain, R.; Kadir, M.R.A. Quinone-rich polydopamine functionalization of yttria stabilized zirconia for apatite biomineralization: The effects of coating temperature. Appl. Surf. Sci. 2015, 346, 317–328. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Yang, D.L.; Zhang, S.H.; Wang, J.; Jian, X.G. Preparation and characterization of poly(phthalazinone ether sulfone ketone) hollow fiber ultrafiltration membranes with excellent thermal stability. J. Membr. Sci. 2006, 280, 957–968. [Google Scholar] [CrossRef]
- Yu, G.P.; Liu, C.; Wang, J.Y.; Xu, J.; Jian, X.G. Synthesis and characterization of poly(arylene ether s-triazine)s containing alkyl-, aryl- and chloro-substituted phthalazinone moieties in the main chain. Polym. Int. 2010, 59, 1233–1239. [Google Scholar] [CrossRef]
- Huang, L.W.; Arena, J.T.; Manickam, S.S.; Jiang, X.Q.; Willis, B.G.; McCutcheon, J.R. Improved mechanical properties and hydrophilicity of electrospun nanofiber membranes for filtration applications by dopamine modification. J. Membr. Sci. 2014, 460, 241–249. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with polydopamine: Enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar] [CrossRef]
- Amstad, E.; Gillich, T.; Bilecka, I.; Textor, M.; Reimhult, E. Ultrastable iron oxide nanoparticle colloidal suspensions using dispersants with catechol-derived anchor groups. Nano Lett. 2009, 9, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Luo, J.Q.; Lv, Y.; Shen, P.; Xu, Z.K. Surface engineering of polymer membranes via mussel-inspired chemistry. J. Membr. Sci. 2015, 483, 42–59. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Statz, A.R.; Rho, J.; Park, T.G.; Messersmith, P.B. Substrate-independent layer-by-layer assembly by using mussel-adhesive-inspired polymers. Adv. Mater. 2008, 20, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; Zhu, L.P.; Zhu, L.J.; Zhu, B.K.; Xu, Y.Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.C.; Wu, J.; Dinh, N.D.; Chen, C.H. Gradient porous elastic hydrogels with shape-memory property and anisotropic responses for programmable locomotion. Adv. Funct. Mater. 2015, 25, 7272–7279. [Google Scholar] [CrossRef]
- Xu, F.J.; Wang, Z.H.; Yang, W.T. Surface functionalization of polycaprolactone films via surface-initiated atom transfer radical polymerization for covalently coupling cell-adhesive biomolecules. Biomaterials 2010, 31, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Park, J.; La, W.G.; Jang, H.K.; Lee, M.; Kim, B.S. 3,4-dihydroxyphenylalanine-assisted hydroxyapatite nanoparticle coating on polymer scaffolds for efficient osteoconduction. Tissue Eng. Part C Methods 2012, 18, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.H.; Deng, Y.; Ye, Z.Y.; Liang, S.S.; Tang, Z.H.; Wei, S.C. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloid Surface B 2013, 111, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Song, J.L.; Ji, P.; Zhang, X.H.; Li, X.M.; Xu, X.; Wang, M.K.; Zhang, S.Q.; Deng, Y.; Deng, F.; et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl. Mater. Int. 2016, 8, 3499–3515. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; He, S.; Wu, X.M.; Liang, S.S.; Mu, Z.L.; Wei, J.; Deng, F.; Deng, Y.; Wei, S.C. Polyetheretherketone/nano-fluorohydroxyapatite composite with antimicrobial activity and osseointegration properties. Biomaterials 2014, 35, 6758–6775. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Li, X.K.; Zhang, X.; Wang, C.R.; Meng, X.F.; Pei, Y.F.; Fan, X.L.; Lan, P.H.; Wang, C.H.; et al. Improving osteointegration and osteogenesis of three-dimensional porous Ti6Ai4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl. Mater. Int. 2015, 7, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.Y.; Teoh, S.H. Surface modification of ultra thin poly (epsilon-caprolactone) films using acrylic acid and collagen. Biomaterials 2004, 25, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, K.; Zhao, R.; Ye, X.J.; Chen, X.N.; Xiao, Z.W.; Yang, X.; Zhu, X.D.; Zhang, K.; Fan, Y.J.; et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials 2017, 147, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Sng, K.B.C.; Li, J. Synthesis and water-swelling of thermo-responsive poly(ester urethane)s containing poly(epsilon-caprolactone), poly(ethylene glycol) and poly(propylene glycol). Biomaterials 2008, 29, 3185–3194. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, W.T.; Cui, J.; Li, X.; Dou, Y.; Su, L.; Chang, J.; Wang, H.J.; Li, X.D.; Zhang, B.B. pH- and NIR light responsive nanocarriers for combination treatment of chemotherapy and photodynamic therapy. Biomater. Sci. 2016, 4, 338–345. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








| Sample a | Contact Angle (deg) | Surface-Energy Components (mN/m) | |||
|---|---|---|---|---|---|
| Water | Diiodomethane | σs a | σs b | σs c | |
| Pristine PPBES | 73.2 ± 2.7 | 48 ± 1.2 | 38.3 | 28.4 | 9.9 |
| PDA-coated PPBES(RT24) | 64.3 ± 1.0 | 44.7 ± 0.8 | 43.1 | 28.1 | 15.0 |
| PDA-coated PPBES(RT48) | 59.0 ± 1.3 | 52.9 ± 1.4 | 43.9 | 22.4 | 21.5 |
| PDA-coated PPBES(RT72) | 58.9 ± 1.5 | 53.9 ± 1.8 | 43.8 | 22.9 | 17.8 |
| PDA-coated PPBES(4548) | 56.0 ± 0.5 | 52.7 ± 1.6 | 45.9 | 22.0 | 23.9 |
| PDA-coated PPBES(6048) | 56.7 ± 0.7 | 60.0 ± 0.6 | 44.2 | 18.1 | 26.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, Y.; Wang, J.; Liu, C.; Liu, W.; Jian, X. Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application. Molecules 2018, 23, 1643. https://doi.org/10.3390/molecules23071643
Liu C, Li Y, Wang J, Liu C, Liu W, Jian X. Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application. Molecules. 2018; 23(7):1643. https://doi.org/10.3390/molecules23071643
Chicago/Turabian StyleLiu, Chengde, Yizheng Li, Jinyan Wang, Cheng Liu, Wentao Liu, and Xigao Jian. 2018. "Improving Hydrophilicity and Inducing Bone-Like Apatite Formation on PPBES by Polydopamine Coating for Biomedical Application" Molecules 23, no. 7: 1643. https://doi.org/10.3390/molecules23071643