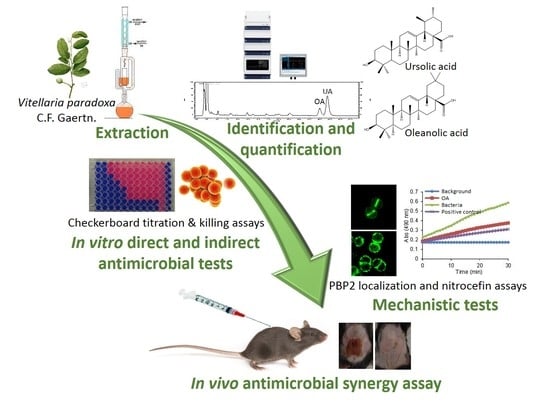
Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analysis and Quantification of Major Components of the Vitellaria paradoxa Crude Extract
2.2. Antimicrobial Activity of the Crude Extract’s Major Components
2.3. Effect of OA and UA on PBP2 Recruitment to the Septum
2.4. Evaluation of β-Lactamase Activity
2.5. Murine Model of Subcutaneous Infection
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Extracts
3.3. Reference Compounds
3.4. UA and OA Identification
3.5. UA and OA Quantification
3.6. Bacteria Strains
3.7. Minimal Inhibitory Concentration (MIC) Determination
3.8. Fractional Inhibitory Concentration Indices (FICI) Determination
3.9. Time-Kill Assay
3.10. Effect of Compounds on PBP2 Localization
3.11. Effect of Compounds on PBP2 and PBP2A Expression
3.12. Evaluation of β-Lactamase Activity using the Nitrocefin Test
3.13. Murine Model of Subcutaneous Infection
3.14. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hall, J.B.; Aebischer, D.P.; Tomlinson, H.F.; Osei-Amaning, E.; Hindle, J.R. Vitellaria Paradoxa: A Monograph.; School of Agricultural and Forest Sciences, University of Wales Bangor: North Wales, UK, 1996; pp. 69–72. [Google Scholar]
- Ayankunle, A.A.; Kolawole, O.T.; Adesokan, A.A.; Akiibinu, M.O. Antibacterial Activity and Sub-chronic Toxicity Studies of Vitellaria paradoxa Stem Bark Extract. J. Pharmacol. Toxicol. 2012, 7, 298–304. [Google Scholar] [CrossRef]
- Jiofack, T.; Fokunang, C.; Guedje, N.; Kemeuze, V.; Fongnzossie, E.; Nkongmeneck, B.A.; Mapongmetsem, P.M.; Tsabang, N. Ethnobotanical uses of medicinal plants of two ethnoecological regions of Cameroon. Int. J. Med. Med. Sci. 2010, 2, 60–79. [Google Scholar]
- Tagne, R.S.; Telefo, B.P.; Nyemb, J.N.; Yemele, D.M.; Njina, S.N.; Goka, S.M.C.; Lienou, L.L.; Nwabo Kamdje, A.H.; Moundipa, P.F.; Farooq, A.D. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac. J. Trop. Med. 2014, 7, S442–S447. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of Antimicrobial Resistance in Bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E. New therapeutic choices for infections caused by methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2009, 15, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Ogunwande, I.A.; Bello, M.O.; Olawore, O.N.; Muili, K.A. Phytochemical and antimicrobial studies on Butyrospermum paradoxum. Fitoterapia 2001, 72, 54–56. [Google Scholar] [CrossRef]
- Catteau, L.; Van Bambeke, F.; Quetin-Leclercq, J. Preliminary evidences of the direct and indirect antimicrobial activity of 12 plants used in traditional medicine in Africa. Phytochem. Rev. 2015, 1–17. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Nyaa, L.B.T.; Tane, P.; Ricciutelli, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ponou, B.K.; Barboni, L. Cytotoxic and antioxidant triterpene saponins from Butyrospermum parkii (Sapotaceae). Carbohydr. Res. 2011, 346, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Talla, E.; Nyemb, J.N.; Tchinda Tiabou, A.; Zambou Djou, S.; Biyanzi, P.; Sophie, L.; Vander Elst, L.; Mbafor Tanyi, J. Antioxidant Activity and a New Ursane-type Triterpene from Vitellaria paradoxa (Sapotaceae) Stem Barks. Eur. J. Med. Plants 2016, 16, 1–20. [Google Scholar] [CrossRef]
- Barber, M.; Waterworth, P.M. Penicillinase-resistant Penicillins. Br. Med. J. 1964, 2, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Kalant, H. The Pharmacology of Semisynthetic Antibiotics. Can. Med. Assoc. J. 1965, 93, 839–843. [Google Scholar] [PubMed]
- EUCAST (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial Activity of Oleanolic and Ursolic Acids: An Update. Evid. Based Complement. Alternat. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.G.; Errington, J. Recruitment of penicillin-binding protein PBP2 to the division site of Staphylococcus aureus is dependent on its transpeptidation substrates. Mol. Microb. 2005, 55, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Lee, S.; Yoon, Y.; Choi, K.-H. Antimicrobial Action of Oleanolic Acid on Listeria monocytogenes, Enterococcus faecium, and Enterococcus faecalis. PLoS ONE 2015, 10, e0118800. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.G.; Filipe, S.R.; de Lencastre, H.; Tomasz, A. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J. Bacteriol. 2001, 183, 6525–6531. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.; Therien, A.G.; Lu, J.; Lee, S.H.; Caron, A.; Gill, C.J.; Lebeau-Jacob, C.; Benton-Perdomo, L.; Monteiro, J.M.; Pereira, P.M.; et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics. Sci. Transl. Med. 2012, 4, 126ra35. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.R.; Monteiro, J.M.; Memmi, G.; Thanassi, J.; Pucci, M.; Schwartzman, J.; Pinho, M.G.; Cheung, A.L. Characterization of a novel small molecule that potentiates β-lactam activity against Gram-positive and Gram-negative pathogens. Antimicrob. Agents Chemother. 2015, 59, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Zussman, J.; Donegan, N.P.; Ramos, R.I.; Garcia, N.C.; Uslan, D.Z.; Iwakura, Y.; Simon, S.I.; Cheung, A.L.; Modlin, R.L.; et al. Noninvasive In Vivo Imaging to Evaluate Immune Responses and Antimicrobial Therapy against Staphylococcus aureus and USA300 MRSA Skin Infections. J. Investig. Dermatol. 2011, 131, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Lemaire, S.; Pinho, M.G.; Mobashery, S.; Hinds, J.; Taylor, P.W. Insertion of epicatechin gallate into the cytoplasmic membrane of methicillin-resistant Staphylococcus aureus disrupts penicillin-binding protein (PBP) 2a-mediated beta-lactam resistance by delocalizing PBP2. J. Biol. Chem. 2010, 285, 24055–24065. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The β-lactam-resistance modifier (–)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Jahangir, M.; Hussan, S.S.; Choudhary, M.I. Inhibition of α-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 2002, 60, 295–299. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Hu, Z.-Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Okumura, C.Y.; Thienphrapa, W.; Olson, J.; Nonejuie, P.; Dam, Q.; Dhand, A.; Pogliano, J.; Yeaman, M.R.; Hensler, M.E.; et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J. Mol. Med. 2014, 92, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Infectious Diseases Society of America Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M.; Petermann, G.W.; et al. Infectious Diseases Society of America, null 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 2015, 61, e26–e46. [Google Scholar] [CrossRef] [PubMed]
- Van Sorge, N.M.; Beasley, F.C.; Gusarov, I.; Gonzalez, D.J.; von Köckritz-Blickwede, M.; Anik, S.; Borkowski, A.W.; Dorrestein, P.C.; Nudler, E.; Nizet, V. Methicillin-resistant Staphylococcus aureus Bacterial Nitric-oxide Synthase Affects Antibiotic Sensitivity and Skin Abscess Development. J. Biol. Chem. 2013, 288, 6417–6426. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Shen, H.-I.; Liu, F.-C.; Tsai, H.-I.; Wu, Y.-C.; Chang, F.-R.; Yu, H.-P. Ursolic acid inhibits superoxide production in activated neutrophils and attenuates trauma-hemorrhage shock-induced organ injury in rats. PLoS ONE 2014, 9, e111365. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, C.; Hwang, S.W.; Im, J.P.; Kim, J.S. Ursolic acid inhibits nuclear factor-κB signaling in intestinal epithelial cells and macrophages, and attenuates experimental colitis in mice. Life Sci. 2014, 110, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Fragoso, T.N.; Yamamoto, E.S.; Laurenti, M.D.; Silva, M.S.; Ferreira, A.F.; Lago, J.H.G.; Gomes, G.S.; Passero, L.F.D. Therapeutic effect of ursolic acid in experimental visceral leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Cornejo Garrido, J.; Chamorro, G.; Garduno Siciliano, L.; Hernandez Pando, R.; Jiménez-Arellanes, A. Acute and subacute toxicity (28 days) of a mixture of ursolic acid and oleanolic acid obtained from Bouvardia ternifolia in mice. Bol. Latinoamer. Caribe Plant. Med. Arom. 2012, 11, 91–102. [Google Scholar]
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña Ortega, N. Effect of Ursolic Acid on Metabolic Syndrome, Insulin Sensitivity, and Inflammation. J. Med. Food 2017, 20, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors. BioMed Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Bland, J.S.; Katke, J.; Darland, G.; Hall, A.; Lerman, R.H.; Lamb, J.; Carroll, B.; Tripp, M. Clinical safety and efficacy of NG440: A novel combination of rho iso-alpha acids from hops, rosemary, and oleanolic acid for inflammatory conditions. Can. J. Physiol. Pharmacol. 2007, 85, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.; Nadir, M.; Mehmood, N.; Farooq, A. In vitro effectiveness of triterpenoids and their synergistic effect with antibiotics against Staphylococcus aureus strains. Indian J. Pharmacol. 2016, 48, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Paulet, D.; Coqueiro, A.; Malheiro, J.; Borges, A.; Saavedra, M.J.; Choi, Y.H.; Simões, M. Antibiotic adjuvants from Buxus sempervirens to promote effective treatment of drug-resistant Staphylococcus aureus biofilms. RSC Adv. 2016, 6, 95000–95009. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Mandalari, G.; Galati, E.M. Norfloxacin and ursolic acid: In vitro association and postantibiotic effect against Staphylococcus aureus. Lett. Appl. Microbiol. 2011, 53, 193–197. [Google Scholar] [CrossRef] [PubMed]
- PHARMEUROPA Common Selfheal fruit-spike. Reference PA/PH/Exp. TCM/T (14) 69 ANP. PHARMEUROPA 2015, 28.
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Document MS100-S23; CLSI: Wayne, PA, USA, 2013. [Google Scholar]
- Eliopoulos, G. Moellering Antimicrobial combinations. In Antibiotics in Laboratory Medicine; Lorian, V., Ed.; the Williams & Wilkins Co: Baltimore, MD, USA, 1996; pp. 330–396. [Google Scholar]
- Bonapace, C.R.; Bosso, J.A.; Friedrich, L.V.; White, R.L. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002, 44, 363–366. [Google Scholar] [CrossRef]
- Haste, N.M.; Hughes, C.C.; Tran, D.N.; Fenical, W.; Jensen, P.R.; Nizet, V.; Hensler, M.E. Pharmacological Properties of the Marine Natural Product Marinopyrrole A against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.M.; Fernandes, P.B.; Vaz, F.; Pereira, A.R.; Tavares, A.C.; Ferreira, M.T.; Pereira, P.M.; Veiga, H.; Kuru, E.; Van Nieuwenhze, M.S.; et al. Cell shape dynamics during the staphylococcal cell cycle. Nat. Commun. 2015, 6, 9055. [Google Scholar] [CrossRef] [PubMed]
- Atilano, M.L.; Pereira, P.M.; Yates, J.; Reed, P.; Veiga, H.; Pinho, M.G.; Filipe, S.R. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2010, 107, 18991–18996. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Reed, P.; Veiga, H.; Pinho, M.G. The Holliday junction resolvase RecU is required for chromosome segregation and DNA damage repair in Staphylococcus aureus. BMC Microbiol. 2013, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- O′Callaghan, C.H.; Morris, A.; Kirby, S.M.; Shingler, A.H. Novel Method for Detection of β-Lactamases by Using a Chromogenic Cephalosporin Substrate. Antimicrob. Agents Chemother. 1972, 1, 283–288. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





| Strain | MIC (mg/L) | FICI | ||||||
|---|---|---|---|---|---|---|---|---|
| UA | OA | Ampicillin | Oxacillin | Ampicillin-UA | Ampicillin-OA | Oxacillin-UA | Oxacillin-OA | |
| ATCC 33591 | 16 | 32 | 64 | 32 | 0.38–1 | 0.31–1 | 0.25–1 | 0.16–1 |
| VUB 2 | 8 | 64 | 32 | 64 | 0.50–1 | 0.25–1 | 0.31–1 | 0.18–1 |
| VUB 10 | 8 | 64 | 128 | 128 | 0.37–1 | 0.31–1 | 0.31–1 | 0.18–1 |
| VUB 20 | 8 | 64 | 64 | 256 | 0.37–1 | 0.25–1 | 0.50–1 | 0.37–1 |
| VUB 30 | 16 | 128 | 32 | 512 | 0.56–1 | 0.37–1 | 0.50–1 | 0.25–1 |
| Compound | MIC (mg/L) | FICI | |
|---|---|---|---|
| OXA | NAF | ||
| UA | 64 | 0.31–1 | 0.28–1 |
| OA | >128 | 0.50–1 | 0.56–1 |
| OXA | 32 | - | - |
| NAF | 32 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catteau, L.; Reichmann, N.T.; Olson, J.; Pinho, M.G.; Nizet, V.; Van Bambeke, F.; Quetin-Leclercq, J. Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms. Molecules 2017, 22, 2245. https://doi.org/10.3390/molecules22122245
Catteau L, Reichmann NT, Olson J, Pinho MG, Nizet V, Van Bambeke F, Quetin-Leclercq J. Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms. Molecules. 2017; 22(12):2245. https://doi.org/10.3390/molecules22122245
Chicago/Turabian StyleCatteau, Lucy, Nathalie T. Reichmann, Joshua Olson, Mariana G. Pinho, Victor Nizet, Françoise Van Bambeke, and Joëlle Quetin-Leclercq. 2017. "Synergy between Ursolic and Oleanolic Acids from Vitellaria paradoxa Leaf Extract and β-Lactams against Methicillin-Resistant Staphylococcus aureus: In Vitro and In Vivo Activity and Underlying Mechanisms" Molecules 22, no. 12: 2245. https://doi.org/10.3390/molecules22122245