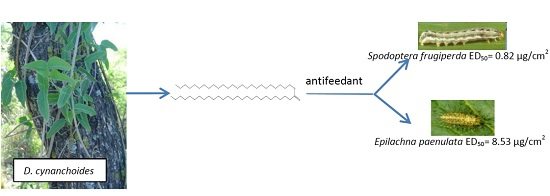
Insecticidal Properties of a Highly Potent Wax Isolated from Dolichandra cynanchoides Cham
Abstract
:1. Introduction
2. Results and Discussion
2.1. Choice Assay against S. frugiperda and E. paenulata
2.2. No-Choice and Mortality Assays against S. frugiperda
2.3. No-Choice and Mortality Assays against E. paenulata
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedures and Apparatus
3.3. Chemicals and Chromatographic Absorbents
3.4. Insects
3.5. Feeding Choice Assay
3.6. No-Choice Feeding Assay
3.7. Mortality Assay
3.8. Isolation of Pentacosyl Heptacosanoate (1)
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González-Coloma, A.; Reina, M.; Gutiérrez, C.; Fraga, B.M. Natural insecticides: Structure diversity, effects and structure-activity relationships. A case study. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 26, Part G; pp. 849–879. [Google Scholar]
- Céspedes, C.L.; Molina, S.C.; Muñoz, E.; Lamilla, C.; Alarcon, J.; Palacios, S.M.; Carpinella, M.C.; Avila, J.G. The insecticidal, molting disruption and insect growth inhibitory activity of extracts from Condalia microphylla cav. (rhamnaceae). Ind. Crops Prod. 2013, 42, 78–86. [Google Scholar] [CrossRef]
- Palacios, S.M.; Maggi, M.E.; Bazán, C.M.; Carpinella, M.C.; Turco, M.; Muñoz, A.; Alonso, R.A.; Nuñez, C.; Cantero, J.J.; Defago, M.T.; et al. Screening of argentinian plants for pesticide activity. Fitoterapia 2007, 78, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Del Corral, S.; Diaz Napal, G.N.; Zaragoza, M.H.; Carpinella, M.C.; Ruiz, G.M.; Palacios, S.M. Screening for extracts with insect antifeedant properties in native plants from central argentina. Bol. Latinoamer. Caribe Plant. Med. Aromat. 2014, 13, 498–505. [Google Scholar]
- Barboza, G.E. Flora Medicinal de la provincia de Córdoba (Argentina): Pteridófitas Y Antófitas Silvestres O Naturalizadas; Museo Botánico: Córdoba, Spain, 2006. [Google Scholar]
- Castillo, L.; González-Coloma, A.; González, A.; Díaz, M.; Santos, E.; Alonso-Paz, E.; Bassagoda, M.J.; Rossini, C. Screening of uruguayan plants for deterrent activity against insects. Ind. Crops Prod. 2009, 29, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Espelie, K.E. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 1995, 40, 171–194. [Google Scholar] [CrossRef]
- Legal, L.; Moulin, B.; Jallon, J.M. The relation between structures and toxicity of oxygenated aliphatic compounds homologous to the insecticide octanoic acid and the chemotaxis of two species of Drosophila. Pestic. Biochem. Physiol. 1999, 65, 90–101. [Google Scholar] [CrossRef]
- Castillo, L.; Díaz, M.; González-Coloma, A.; González, A.; Alonso-Paz, E.; Bassagoda, M.J.; Rossini, C. Clytostoma callistegioides (bignoniaceae) wax extract with activity on aphid settling. Phytochemistry 2010, 71, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Pillai, S.K. Neonate Plutella xylostella responses to surface wax components of a resistant cabbage (Brassica oleracea). J. Chem. Ecol. 1998, 24, 1611–1627. [Google Scholar] [CrossRef]
- Pande, A.; Shukla, Y.N.; Tripathi, A.K. Lipid constituents from Stellaria media. Phytochemistry 1995, 39, 709–711. [Google Scholar] [CrossRef]
- Bonini, C.; Davini, E.; Iavarone, C.; Trogolo, C. Cynanchoside, a highly oxygenated iridoid glucoside from Macfadyena cynanchoides. Phytochemistry 1981, 20, 1587–1590. [Google Scholar] [CrossRef]
- Adriani, C.; Iavarone, C.; Trogolo, C. 5,7-bisdeoxycynanchoside, an iridoid glucoside from Macfadyena cynanchoides. Phytochemistry 1982, 21, 231–233. [Google Scholar] [CrossRef]
- Dobler, S.; Petschenka, G.; Pankoke, H. Coping with toxic plant compounds—The insect’s perspective on iridoid glycosides and cardenolides. Phytochemistry 2011, 72, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Koul, O. Insect Antifeedants; CRC Press LLC: Boca Raton, FL, USA, 2005. [Google Scholar]
- Eigenbrode, S.D.; Jetter, R. Attachment to plant surface waxes by an insect predator. Integr. Comp. Biol. 2002, 42, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Riederer, M. Plant surface properties in chemical ecology. J. Chem. Ecol. 2005, 31, 2621–2651. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Espelie, K.E.; Todd, J.W.; Culbreath, A.K.; Pittman, R.N.; Demski, J.W. Cuticular lipids from wild and cultivated peanuts and the relative resistance of these peanut species to fall armyworm and thrips. J. Agric. Food Chem. 1993, 41, 814–818. [Google Scholar] [CrossRef]
- Yang, G.; Wiseman, B.R.; Isenhour, D.; Espelie, K. Chemical and ultrastructural analysis of corn cuticular lipids and their effect on feeding by fall armyworm larvae. J. Chem. Ecol. 1993, 19, 2055–2074. [Google Scholar] [CrossRef] [PubMed]
- Bergman, D.K.; Dillwith, J.W.; Zarrabi, A.A.; Caddel, J.L.; Berberet, R.C. Epicuticular lipids of alfalfa relative to its susceptibility to spotted alfalfa aphids (homoptera: Aphididae). Environ. Entomol. 1991, 20, 781–785. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Defago, M.T.; Valladares, G.; Palacios, S.M. Antifeedant and insecticide properties of a limonoid from Melia azedarach (meliaceae) with potential use for pest management. J. Agric. Food Chem. 2003, 51, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Diaz Napal, G.N.; Defagó, M.; Valladares, G.; Palacios, S.M. Response of Epilachna paenulata to two flavonoids, pinocembrin and quercetin, in a comparative study. J. Chem. Ecol. 2010, 36, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.E.; Mangeaud, A.; Carpinella, M.C.; Ferrayoli, C.G.; Valladares, G.R.; Palacios, S.M. Laboratory evaluation of Artemisia annua L. Extract and artemisinin activity against Epilachna paenulata and Spodoptera eridania. J. Chem. Ecol. 2005, 31, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.N.P.; Neeharika, T.S.V.R.; Kumar, T.P.; Satyavathi, B.; Sailu, C.; Prasad, R.B.N. Kinetics of enzymatic esterification of oleic acid and decanol for wax ester and evaluation of its physico-chemical properties. J. Taiwan Inst. Chem. Eng. 2015, 55, 12–16. [Google Scholar] [CrossRef]
- Bianchi, G.; Murelli, C.; Vlahov, G. Surface waxes from olive fruits. Phytochemistry 1992, 31, 3503–3506. [Google Scholar] [CrossRef]
- Palacios, C.E.J.; Negri, G.; Salatino, A. Esters and other constituents of the foliar cuticular wax of a soybean variety. Biochem. Syst. Ecol. 2015, 63, 198–200. [Google Scholar] [CrossRef]
- Kanya, T.C.S.; Rao, L.J.; Sastry, M.C.S. Characterization of wax esters, free fatty alcohols and free fatty acids of crude wax from sunflower seed oil refineries. Food Chem. 2007, 101, 1552–1557. [Google Scholar]
- Diaz Napal, G.; Palacios, S. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Defagó, M.; Valladares, G.; Banchio, E.; Carpinella, M.C.; Palacios, S.M. Insecticide and antifeedant activity of different plant parts of Melia azedarach on Xanthogaleruca luteola. Fitoterapia 2006, 77, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Mazoir, N.; Benharref, A.; Bailén, M.; Reina, M.; González-Coloma, A. Bioactive triterpene derivatives from latex of two Euphorbia species. Phytochemistry 2008, 69, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.






| Compound | Insect | ED50 (µg/cm2) (Values and 95% Confidence Interval) |
|---|---|---|
| Pentacosyl heptacosanoate (1) | S. frugiperda | 0.82 (0.15–4.50) |
| Pentacosyl heptacosanoate (1) | E. paenulata | 8.53 (2.73–26.59) |
| Azadirachtin (2) | S. frugiperda | 0.10 (0.05–0.19) |
| Azadirachtin (2) | E. paenulata | 0.59 (0.07–4.37) |
| Dosage µg/cm2 | LT50 in Days (Values and 95% Confidence Interval) a | |
|---|---|---|
| 1 | Food-Deprived Larvae | |
| Spodoptera frugiperda | ||
| 50 | 3.14 (2.88–6.40) | |
| 5 | 3.31 (3.10–6.70) | |
| 1 | 6.96 (3.62–16.00) | |
| 0.1 | 7.71 (4.81–16.53) | |
| 3.16 (2.63–6.03) | ||
| Epilachna paenulata | ||
| 50 | 4.69 (2.78–7.80) | |
| 5 | 7.56 (5.62–9.96) | |
| 1 | 7.80 (4.89–10.06) | |
| 0.1 | 9.62 (7.06–13.13) | |
| 4.10 (2.59–6.19) | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz Napal, G.; Carpinella, M.C.; Palacios, S.M. Insecticidal Properties of a Highly Potent Wax Isolated from Dolichandra cynanchoides Cham. Molecules 2016, 21, 1039. https://doi.org/10.3390/molecules21081039
Díaz Napal G, Carpinella MC, Palacios SM. Insecticidal Properties of a Highly Potent Wax Isolated from Dolichandra cynanchoides Cham. Molecules. 2016; 21(8):1039. https://doi.org/10.3390/molecules21081039
Chicago/Turabian StyleDíaz Napal, Georgina, María C. Carpinella, and Sara M. Palacios. 2016. "Insecticidal Properties of a Highly Potent Wax Isolated from Dolichandra cynanchoides Cham" Molecules 21, no. 8: 1039. https://doi.org/10.3390/molecules21081039