Effect of Drying Methods on the Steroidal Alkaloid Content of Potato Peels, Shoots and Berries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Distribution of Steroidal Alkaloids in Different Parts of Potato Plant
2.2. Changes in Steroidal Alkaloid Content during Air Drying of Potato Shoots
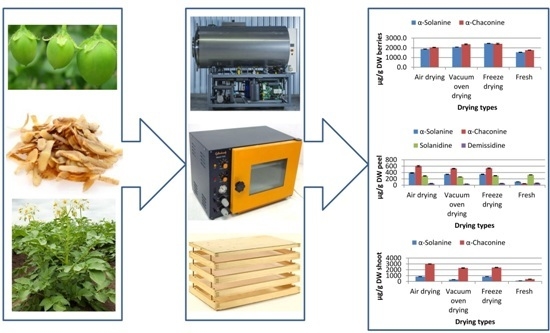
2.3. Effect of Different Drying Methods on Steroidal Alkaloid Content of Different Plant Parts
3. Experimental Section
3.1. Samples and Reagents
3.2. Drying of Fresh Potato Plant Parts
3.3. Preparation of Extracts from Fresh Samples
3.4. Preparation of Extracts from Dried Plant Parts
3.5. Identification and Quantification of Steroidal Alkaloids in Potato Peel by Ultra-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schieber, A.; Saldana, M.A. Potato peels: A source of nutritionally and pharmacologically interesting compounds—A review. Food 2009, 3, 23–29. [Google Scholar]
- Morris, S.; Lee, T. The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): A review. Food Technol. Aust. 1984, 36, 118–124. [Google Scholar]
- Slanina, P. Solanine (glycoalkaloids) in potatoes: Toxicological evaluation. Food Chem. Toxicol. 1990, 28, 759–761. [Google Scholar] [CrossRef]
- Hu, K.; Kobayashi, H.; Dong, A.; Jing, Y.; Iwasaki, S.; Yao, X. Antineoplastic Agents III: Steroidal Glycosides from Solanum nigrum. Planta Medica 1999, 65, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, R.; Kenny, O.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Jones, P.W.; O’Brien, N.; Maguire, A.R.; Collins, S.G. Synthesis of novel 24-amino-25, 26, 27-trinorlanost-8-enes: Cytotoxic and apoptotic potential in U937 cells. Bioorg. Med. Chem. 2015, 23, 2270–2280. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, R.; Kagan, I.A. In This Issue “Phytoalexins into the 21st century”. Physiol. Mol. Plant Pathol. 2001, 59, 59–61. [Google Scholar] [CrossRef]
- Friedman, M.; Dao, L. Distribution of glycoalkaloids in potato plants and commercial potato products. J. Agric. Food Chem. 1992, 40, 419–423. [Google Scholar] [CrossRef]
- Phillips, B.J.; Hughes, J.A.; Phillips, J.C.; Walters, D.G.; Anderson, D.; Tahourdin, C.S. A study of the toxic hazard that might be associated with the consumption of green potato tops. Food Chem. Toxicol. 1996, 34, 439–448. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Griffiths, D.W.; Dale, M.F.B.; Bain, H. The effect of cultivar, maturity and storage on photo-induced changes in the total glycoalkaloid and chlorophyll contents of potatoes (Solanum tuberosum). Plant Sci. 1994, 98, 103–109. [Google Scholar] [CrossRef]
- Machado, R.M.D.; Toledo, M.C.F.; Garcia, L.C. Effect of light and temperature on the formation of glycoalkaloids in potato tubers. Food Control 2007, 18, 503–508. [Google Scholar] [CrossRef]
- Percival, G.C. The influence of light upon glycoalkaloid and chlorophyll accumulation in potato tubers (Solanum tuberosum L.). Plant Sci. 1999, 145, 99–107. [Google Scholar] [CrossRef]
- Rosenfeld, H.J.; Sundell, H.A.; Lea, P.; Ringstad, M. Influence of packaging materials and temperature on the glycoalkaloid content of potato tubers. Food Res. Int. 1995, 28, 481–484. [Google Scholar] [CrossRef]
- Sengül, M.; Keles, F.; Keles, M.S. The effect of storage conditions (temperature, light, time) and variety on the glycoalkaloid content of potato tubers and sprouts. Food Control 2004, 15, 281–286. [Google Scholar] [CrossRef]
- Caldwell, K.A.; Grosjean, O.K.; Henika, P.R.; Friedman, M. Hepatic ornithine decarboxylase induction by potato glycoalkaloids in rats. Food Chem. Toxicol. 1991, 29, 531–535. [Google Scholar] [CrossRef]
- Friedman, M.; Rayburn, J.R.; Bantle, J.A. Developmental toxicology of potato alkaloids in the frog embryo teratogenesis assay--Xenopus (FETAX). Food Chem. Toxicol. 1991, 29, 537–547. [Google Scholar] [CrossRef]
- Suhaj, M. Spice antioxidants isolation and their antiradical activity: A review. J. Food Compos. Anal. 2006, 19, 531–537. [Google Scholar] [CrossRef]
- Di Cesare, L.F.; Forni, E.; Viscardi, D.; Nani, R.C. Changes in the Chemical Composition of Basil Caused by Different Drying Procedures. J. Agric. Food Chem. 2003, 51, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Yousif, A.N.; Scaman, C.H.; Durance, T.D.; Girard, B. Flavor Volatiles and Physical Properties of Vacuum-Microwave- and Air-Dried Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 1999, 47, 4777–4781. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, N.; Stankovic, M. Hydrolysis of glycoalkaloids from Solanum tuberosum L. haulm by enzymes present in plant material and by enzyme preparation. Potato Res. 2005, 48, 25–33. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P. Optimisation and validation of ultra-high performance liquid chromatographic-tandem mass spectrometry method for qualitative and quantitative analysis of potato steroidal alkaloids. J. Chromatogr. B 2015, 997, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.T.; Vir, S.; Bhutani, K.K. Liquid chromatography-mass spectrometry-based quantification of steroidal glycoalkaloids from Solanum xanthocarpum and effect of different extraction methods on their content. J. Chromatogr. A 2008, 1208, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.





| Sample | Moisture Loss (%) | ||
|---|---|---|---|
| Freeze Drying | Air Drying | Vacuum Oven Drying | |
| Potato peel | 78 ± 0.6 | 75 ± 0.5 | 77 ± 1.1 |
| Shoots | 92 ± 1.0 | 90 ± 1.2 | 91 ± 1.0 |
| Berries | 85 ± 1.5 | 82 ± 0.5 | 83 ± 1.5 |
| Compound | MRM Transitions | Dwell Time (ms) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|
| α-Solanine | m/z 868.54➔m/z 722.30 | 0.042 | 98 | 79 |
| α-Chaconine | m/z 852.53➔ m/z 706.75 | 0.042 | 94 | 76 |
| Solanidine | m/z 398.24➔ m/z 147.42 | 0.042 | 62 | 42 |
| Demissidine | m/z 400.37 ➔ m/z 118.68 | 0.042 | 74 | 72 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.B.; Brunton, N.P.; Rai, D.K. Effect of Drying Methods on the Steroidal Alkaloid Content of Potato Peels, Shoots and Berries. Molecules 2016, 21, 403. https://doi.org/10.3390/molecules21040403
Hossain MB, Brunton NP, Rai DK. Effect of Drying Methods on the Steroidal Alkaloid Content of Potato Peels, Shoots and Berries. Molecules. 2016; 21(4):403. https://doi.org/10.3390/molecules21040403
Chicago/Turabian StyleHossain, Mohammad B., Nigel P. Brunton, and Dilip K. Rai. 2016. "Effect of Drying Methods on the Steroidal Alkaloid Content of Potato Peels, Shoots and Berries" Molecules 21, no. 4: 403. https://doi.org/10.3390/molecules21040403